CHM 151 Exp #8 Copper Cycle General Observations: Your instructor has provided pictures and video taken during each of the five different steps (reactions) during this experiment. Please refer to them as you write out your general observations for the experiment. For example: Table 1- General Observations of the Copper Reactions in this experiment Step 1: Cu(s) + 4HNO3(aq) → Cu(NO3);(aq) + 2NO:(g) + 2H;O(I) Reddish brown gas is produced and the copper dissolves to form a blue solution. The reaction is producing heat (beaker is getting hotter). Since there are five steps in this series there should be general observations written down for each reaction (step) in the series (5). It is important to record observations of color, gas production, precipitate production, dissolving of solids and changes of temperature since these types of general observations are all indicators of a chemical reaction taking place. You will find numbers written in parentheses for the questions placed in the procedure corresponding to the questions in the question section. You may find it more convenient to answer these questions as you are working through the different reactions in the lab, rather than trying to go back and remember what was happening later. Data Section: The following items should be included in the data section in your lab notebook and any lab report. (1) General Observations should be recorded – what did you observe during the experiment? (2) Write the balanced chemical equation for this reaction. (3) Data Table - The masses are from an actual experiment completed on Friday, March 20, 2020. 1. Initial mass of copper 0.4944 g 2. Mass of copper and evaporating dish 48.0337 g 3. Mass of evaporating dish 47.5789 g 4. Mass of recovered copper 0.4548 g
CHM 151 Exp #8 Copper Cycle General Observations: Your instructor has provided pictures and video taken during each of the five different steps (reactions) during this experiment. Please refer to them as you write out your general observations for the experiment. For example: Table 1- General Observations of the Copper Reactions in this experiment Step 1: Cu(s) + 4HNO3(aq) → Cu(NO3);(aq) + 2NO:(g) + 2H;O(I) Reddish brown gas is produced and the copper dissolves to form a blue solution. The reaction is producing heat (beaker is getting hotter). Since there are five steps in this series there should be general observations written down for each reaction (step) in the series (5). It is important to record observations of color, gas production, precipitate production, dissolving of solids and changes of temperature since these types of general observations are all indicators of a chemical reaction taking place. You will find numbers written in parentheses for the questions placed in the procedure corresponding to the questions in the question section. You may find it more convenient to answer these questions as you are working through the different reactions in the lab, rather than trying to go back and remember what was happening later. Data Section: The following items should be included in the data section in your lab notebook and any lab report. (1) General Observations should be recorded – what did you observe during the experiment? (2) Write the balanced chemical equation for this reaction. (3) Data Table - The masses are from an actual experiment completed on Friday, March 20, 2020. 1. Initial mass of copper 0.4944 g 2. Mass of copper and evaporating dish 48.0337 g 3. Mass of evaporating dish 47.5789 g 4. Mass of recovered copper 0.4548 g
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.57PAE
Related questions
Question
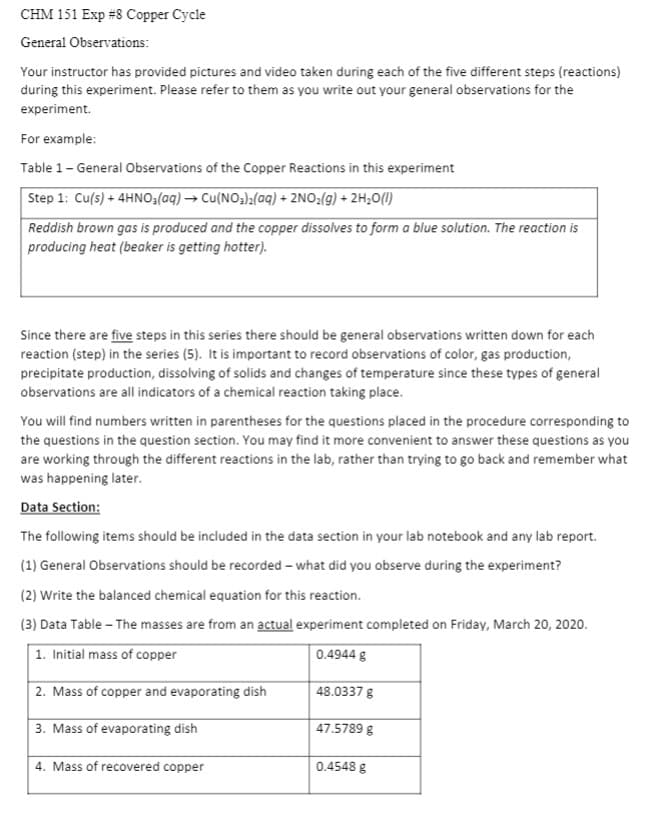
Transcribed Image Text:CHM 151 Exp #8 Copper Cycle
General Observations:
Your instructor has provided pictures and video taken during each of the five different steps (reactions)
during this experiment. Please refer to them as you write out your general observations for the
experiment.
For example:
Table 1- General Observations of the Copper Reactions in this experiment
Step 1: Cu(s) + 4HNO;(aq) → Cu(NO3);(aq) + 2NO:(g) + 2H;O()
Reddish brown gas is produced and the copper dissolves to form a blue solution. The reaction is
producing heat (beaker is getting hotter).
Since there are five steps in this series there should be general observations written down for each
reaction (step) in the series (5). It is important to record observations of color, gas production,
precipitate production, dissolving of solids and changes of temperature since these types of general
observations are all indicators of a chemical reaction taking place.
You will find numbers written in parentheses for the questions placed in the procedure corresponding to
the questions in the question section. You may find it more convenient to answer these questions as you
are working through the different reactions in the lab, rather than trying to go back and remember what
was happening later.
Data Section:
The following items should be included in the data section in your lab notebook and any lab report.
(1) General Observations should be recorded – what did you observe during the experiment?
(2) Write the balanced chemical equation for this reaction.
(3) Data Table – The masses are from an actual experiment completed on Friday, March 20, 2020.
1. Initial mass of copper
0.4944 g
2. Mass of copper and evaporating dish
48.0337 g
3. Mass of evaporating dish
47.5789 g
4. Mass of recovered copper
0.4548 g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning