Choose a heating curve for 1 mole of ethanol beginning at 140 K and ending at 380 K. Assume that the values for ethanol given here are constant over the relevant temperature ranges. Vapour/gas Melting Point 159 K 340 Boiling Point 352 K ΔΗ 4.9 kJ - mol 290 ΔΗ 38.6 kJ - mol 1 Liquid 240 111 J. mol. K ! 1 C solid C, liquid 110 J. mol .K 1 190 Cs, gas 78.3 J- mol .K ' Solid 140 10 20 30 40 50 60 70 80 Heat added (kJ/mol) Vapour/gas 340 290 240 Liquid 190 Solid 140 10 20 30 40 50 60 70 80 Heat added (kJ/mol) Temperature (K) Temperature (K)
Choose a heating curve for 1 mole of ethanol beginning at 140 K and ending at 380 K. Assume that the values for ethanol given here are constant over the relevant temperature ranges. Vapour/gas Melting Point 159 K 340 Boiling Point 352 K ΔΗ 4.9 kJ - mol 290 ΔΗ 38.6 kJ - mol 1 Liquid 240 111 J. mol. K ! 1 C solid C, liquid 110 J. mol .K 1 190 Cs, gas 78.3 J- mol .K ' Solid 140 10 20 30 40 50 60 70 80 Heat added (kJ/mol) Vapour/gas 340 290 240 Liquid 190 Solid 140 10 20 30 40 50 60 70 80 Heat added (kJ/mol) Temperature (K) Temperature (K)
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter6: Equilibria In Single-component Systems
Section: Chapter Questions
Problem 6.41E
Related questions
Question
Need solution urgently
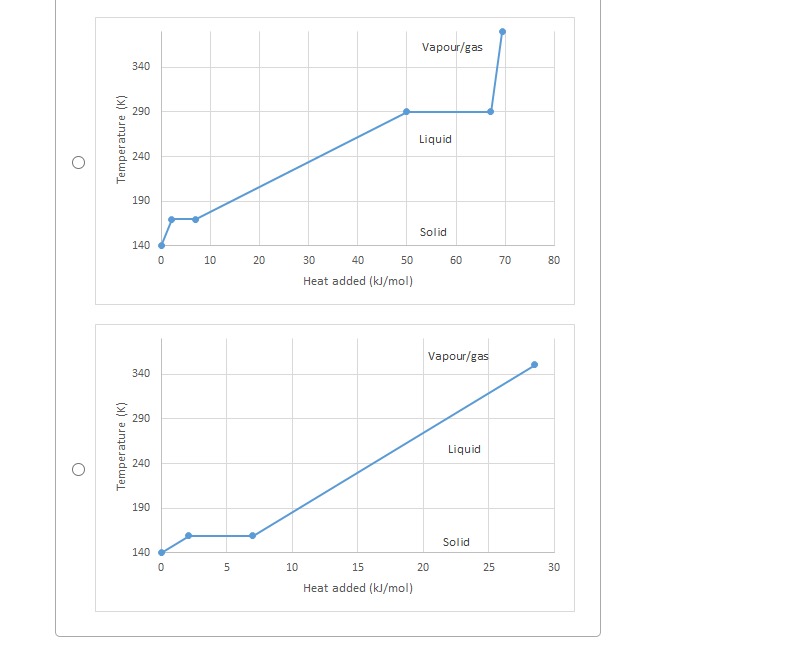
Transcribed Image Text:Vapour/gas
340
290
Liquid
240
190
Solid
140
10
20
30
40
50
60
70
80
Heat added (kJ/mol)
Vapour/gas
340
290
Liquid
240
190
Solid
140
10
15
20
25
30
Heat added (kJ/mol)
Temperature (K)
Temperature (K)
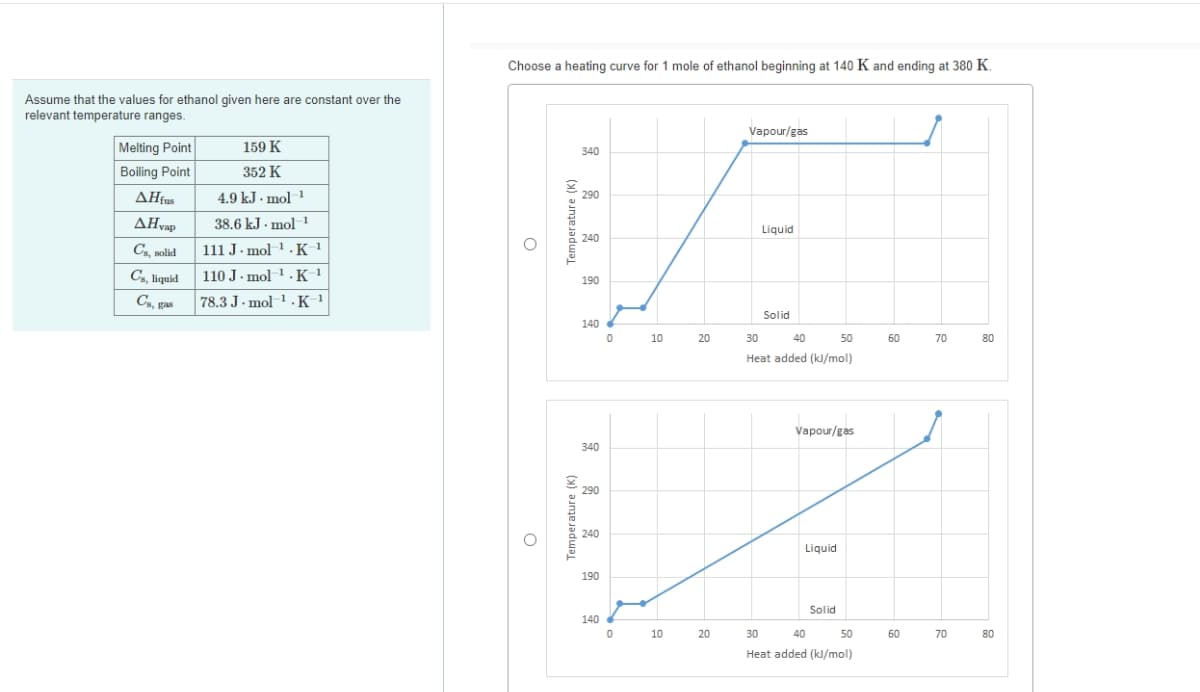
Transcribed Image Text:Choose a heating curve for 1 mole of ethanol beginning at 140 K and ending at 380 K.
Assume that the values for ethanol given here are constant over the
relevant temperature ranges.
Vapour/gas
Melting Point
Boiling Point
ΔΗ
159 K
340
352 K
4.9 kJ - mol-1
290
ΔΗ
38.6 kJ . mol 1
Liquid
240
Cs, solid
111 J· mol 1 . K '
Cs, liquid
110 J. mol 1. K '
190
C, gas
78.3 J. mol 1 .K_1
Solid
140
10
20
30
40
50
60
70
80
Heat added (kJ/mol)
Vapour/gas
340
290
{ 240
Liquid
190
Solid
140
10
20
30
40
50
60
70
80
Heat added (kJ/mol)
Temperature (K)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning