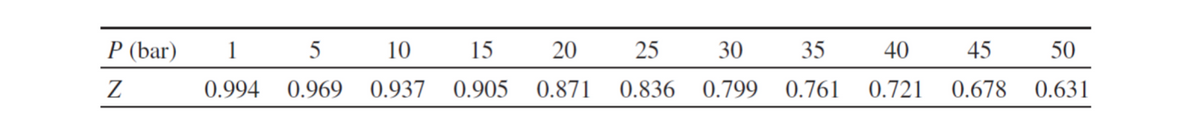
The vapor pressure of n-heptanol is 84.4 kPa at 443 K and its normal boiling point is It is 448K. Estimate the heat of evaporation of N-heptanol
Q: Write ONLY the balanced "NET" ionic equations for each of the reactions. If no net, write NR. barium…
A: In double displacement reaction, Precipitate is formed. In Net Ionic Equation, Common ions on both…
Q: What is the activation energy for a reaction if k = 1.89 × 10–1 s–1 at 541 Kelvin and k = 7.31 s–1…
A: It is based on the Arhenius equation which relate the rate constant With the temperature Here we…
Q: H + Н Н — 2 products
A:
Q: Measurements show that the enthalpy of a mixture of gaseous reactants increases by 214. kJ during a…
A: GIven: The decrease in enthalpy of a reaction = 214 kJ The work done on the mixture = 62 kJ We have…
Q: 15.31. Calculate the pH and pOH of solutions with the following [H₂O*] or [OH-] values. Indicate…
A: “Since you have posted multiple questions, we will provide the solutiononly to the first question(as…
Q: The initial rate of oxidation of sodium succinate to mate the half-life for the reaction form sodium…
A: Given in following question some data table and give some numerical values to use these table…
Q: Q12:- Reaction of HBr with 2-methylpropene yields 2-bromo-2-methylpropane. What is the structure of…
A: Alkene reacts with hydrogen halide to form alkyl halide. It undergo via carbocation intermediate, In…
Q: 15) For the reaction R-P, the concentration of a reactant changes from 0.0300 M to 0.0282 M in 25…
A: NOTE : I HAVE BEEN ASKED TO SOLVE ONLY QUETION NUMBER 15 SO I AM GOING TO TO SOLVE ONLY THAT ,IF YOU…
Q: For the following reaction, write the half-reactions, stating which is the reduction half-reaction…
A: The given redox reaction is 2H+(aq) + H2O2(aq) +2I-(aq) →I2(s) + 2H2O(l) In the reduction half…
Q: Draw the energy diagram for the reaction below showing the transition state (s)
A:
Q: Which is more reducing agent Li or Fr? Why?
A: A substance that itself oxidizes acts as a reducing agent. The power of the reducing agent depends…
Q: Draw the structure of the major organic product of the following reaction. Predict whether the…
A: Carbonyl compounds having at least one alpha hydrogen atom when treated with dilute base it undergo…
Q: Curved arrows are used to illustrate the flow of electrons. Follow the curved arrows and draw the…
A: The lone pairs of the heteroatoms involves in resonance to stabilize the molecule. Generally,…
Q: The standard reaction enthalpy for 2 CO(g) ---> 2 C(s) + O2(g) is: +2 ΔHf°[CO(g)] +…
A:
Q: Calculate the percentage efficiency of extraction of phycocyanin in sample 3, if the amount of…
A: Given, Amount of phycocyanin in sample = 90.7 mg Amount of phycocyanin extracted from sample =…
Q: A three-dimensional representation of butane is shown. Translate the information about the…
A: In Newmann's representation, the conformers are represented in the shape of Y. A circle represents a…
Q: For the d configuration, answer the questions below: Determine the ground term and name the ground…
A:
Q: For the combustion of glucose at 25 °C, determine where the reaction will be more favoured at 37 °C
A: It is given that the combustion for the glucose is at 25 °C and we need to determine where the…
Q: 1. What is the pH of the solution formed by mixing 50 mL of 0.2M Na₂CO3 solution with a) 25 mL of…
A: Note: Since you have posted a question with multiple sub-parts, we will provide the solution only to…
Q: b) If the run took 50 minutes, what is the average power involved? 2. Suppose you get all your…
A:
Q: 2 → ↓ बो OTos olt 14 OTOS
A:
Q: What current will a solar cell with 2.18 m² surface area produce in eight hours? The solar cell is…
A: Given that the surface area of the solar cell is 2.18 m2. Efficiency is 18% Sunlight intensity is…
Q: TEST FOR THE CARBONYL GROUP Name of Test/ Test Samples 2,4 - dinitrophenylhydrazine test…
A: An aqueous solution of 2,4-dinitrophenyl hydrazine (DNP) is known as Brady's reagent
Q: Which of the following conditions would lead to an increase in the relative supersaturation of a…
A: In most cases as we increase the temperature solubility will also increase. As compared to room…
Q: Draw the condensed structural formula for glyceryl tricaprylate-
A:
Q: Consider the following hypothetical aqueous reaction: A(aq) → B(aq). A flask is charged with 0.065…
A:
Q: Which of the following titrant-primary standard pairs is INCORRECT? a. AgNO3-NaCl b. NaOH-KHP c.…
A:
Q: Give explanation Solution
A: A competitive inhibitor competes with a substrate for binding to the active site of an enzyme by…
Q: Classify the statements as either a physical or chemical property of a protein. Chemical property…
A:
Q: Balance this redox reaction in acidic conditions using the half-reaction method. Cd(s) + NO3- (aq)…
A: Redox reactions are chemical processes that involve oxidation and reduction that cause the…
Q: Give examples of sample analyte in a matrix.
A: Please find your solution below : In chemical analysis in analytical chemistry, matrix is defined as…
Q: What is the formula for the molecule shown below?
A: •Here we have to write its Formula. •So firstly we will make its skeletal structure than we count…
Q: The names and gram molecular weights of important compounds involved in this experiment are: Name…
A: Any chemical compound in indicated by a unique way called as chemical formula. In chemical formula…
Q: 200.0 mL of a 3.50 mol/L solution of potassium chloride is added to a concentrated lead (II) acetate…
A: Given, The concentration ( molarity) of the potassium chloride = KCl = 3.50 mol/L The volume of the…
Q: Explain the working principle of following types of distillation, i. Simple Distillation; ii. Steam…
A: “Since you have posted a question with multiple sub-parts, we will solve the first three sub-parts…
Q: Carbon-14 has a half-life of 5730 yr. A living organism has an activity of 15.2 counts per minute…
A:
Q: What is the change in oxidation number of an atom of chromium in the following half-reaction?…
A: Oxidation number: The covalent bond between the atoms of different electronegativity is polar. If we…
Q: Draw the structure of the product of the Diels-Alder reaction below. OCH3 H₂C NC-C=C-CN
A:
Q: 3. Draw the structure of the major organic compound produced when each of the following compounds is…
A: It is based on the concept of neutralization reaction. Here we are required to find the major…
Q: 5. Write a nuclear reaction to describe the following processes. Then calculate the energy change…
A: Nuclear reaction: The given reaction is a nuclear reaction. In the nuclear reaction, one nuclide is…
Q: 2) In the following figure, if ΔΗ, = 1000 KJ, ΔΗ; = +1000 KJ, ΔΗ4 = -1000 KJ, calculate ΔΗ2? a) ΔΗz=…
A: NOTE : I HAVE BEEN ASKED TO SOLVE QUESTION NUMBER 2 ONLY , SO I AM GOING TO SOLVE ONLY THIS IF YOU…
Q: How many electrons are transferred in the following balanced redox reaction? 5NO2- (aq) + 6H+ +…
A:
Q: Which of the following is most suitable for filtering the crystalline precipitate BaSO4 out of the…
A: When two soluble reactants in an aqueous solution are mixed together, an insoluble product may…
Q: Regarding the following reaction: F2 (g) + 2 I^- (aq) → 2 F^- (aq) + I2 (s) a. List the species…
A:
Q: Question 4 of 37 Determine the amount in grams of KCI that exists in 20.3 g of a solution that…
A: Weight of solution= 20.3 gm %KCl by mass = 1.14 %
Q: (a) Which of the following ions is present in the solution if a precipitate was observed with the…
A: Solubility in water refers to the interaction of solute with solvent. Higher the interaction higher…
Q: KMnO4 (aq) + H₂O2 (aq) → MnO2 (aq) + KOH (aq) + H₂O (1) + O2(g) r = AC02/At The initial rate of…
A:
Q: as Match the statement in the left column with the best concept from the right colum. Stereoisomers…
A: Stereoisomers are the isomers that have the same molecular formula and sequence of bonded atoms but…
Q: What are the applications of analytical chemistry?
A: Analytical chemistry deals with identification, detection, characterization and quantification of…
Q: (a) The unit cells of three structural types of ionic! appear light and the cations dark). The…
A: Q-1 A) Anions оссuру Corner + Face • Anions are in Face centered cubic Cations occupy :- Alternate…
The vapor pressure of n-heptanol is 84.4 kPa at 443 K and its normal boiling point is It is 448K. Estimate the heat of evaporation of N-heptanol.
Step by step
Solved in 3 steps

- I am slightly confused on how to do this. I think I figured it out but I just want to compare data and take notes on what I have done incorrectly.In Active Example 3-29 you calculated that you would have to work six weeks to earn enough money to buy a 1082.49 television. You would be working five shifts of four hours each at 9.25/hr. But, alas, when you received your first pay check, you found that exactly 23 of your earnings had been withheld for social security, federal and state income taxes, and workers compensation insurance. Taking these into account, how many weeks will it take to earn the 1082.49?Chemistry Fraction no. Elution Vol. (mL) Abs (λ = 410) Fraction no. Elution Vol. (mL) Abs (λ = 410) 1 (PB pH 6.0) 1 0.0050 11 11 0.0041 2 2 0.0032 12 12 0.0060 3 3 0.0040 13 13 0.0064 4 4 0.0064 14 14 0.0063 5 5 0.0067 15 15 0.1109 6 6 0.0067 16 16 2.8902 7 7 0.0050 17 17 0.8500 8 (PB pH 8.0) 8 0.0049 18 18 0.2019 9 9 0.0052 19 19 0.0378 10 10 0.0050 20 20 0.0073 Use the data above to plot an elution profile, in which the absorbance is on Y-axis, and elution volume (volume = fraction number) is on X-axis. The elution profile must indicate at (1) which point the pH change occurred, (2) the fraction(s) which contain the highest myoglobin content, and (3) the maximum absorbance detected.
- Time(s) [X3] (mole/L) 0 0.600 200 0.458 400 0.362 600 0.281 800 0.204 1000 0.175 1200 0.134 1400 0.104 1600 0.083 1800 0.063 2000 0.047 plot ln[X3] vs time plot 1/[X3] vs timeA. B. and C. already solved (https://www.bartleby.com/questions-and-answers/chemistry-question/a89691d6-2162-4677-b0cc-dc84fcceda08). Please answer E, F and GCircle in pencil in the image on th eirght cide of the diagram is a "2"( Pb(NO3)2 ).a Table: (This is an example of what your data table should look like.) Pressure (kPa) Temperature (°C) Temperature (K) Constant, k(P / T or P•T) Ice water bath 91.58 2.4 275.55 Room temperature 96.54 21.1 294.25 Warm water 104.11 44.5 317.65 Boiling water 113.75 77.5 350.5 Calculations: One way to determine if a relationship is inverse or direct is to find a proportionality constant, k, from the data. If this relationship is direct, k = P/T. If it is inverse, k = P•T. Choose one of these formulas and calculate k for the pairs in your data table (divide or multiply the P and T values). Record the answers in the fourth column of the Data table. How constant were the values for k you obtained in Question 4? Good data may show some minor variation, but the values for k should be relatively constant. Comment on your calculated k values. Analysis Questions: What does temperature measure? Look at your graph. What type…
- 15.4a 3 please. 0.166 was incorrect.Chemistry (i) For T = 298K ΔG° = -8.314 x 298 x ln (2.83x10-3) = 14.524 KJ/mol (ii) For T = 308.15K ΔG° = -8.314 x 308.15 x ln (9.619x10-3) = 11.891 KJ/mol (iii) For T = 318.15K ΔG° = -8.314 x 318.15 x ln (3.14x10-2) = 9.147 KJ/mol (iv) For T = 328.15K ΔG° = -8.314 x 328.15 x ln (8.82x10-2) = 6.621 KJ/mol (v) For T = 338.15K ΔG° = -8.314 x 338.15 x ln (2.42x10-1) = 3.990 KJ/mol Based on the measured ΔG° values, is this equilibrium spontaneous at room temperature? Which factor, entropy, or enthalpy, has the greater impact on spontaneity in this case? Explain your answers.See image below. Can you please show me how to get to 0.00018 as the answer?
- Data obtained: Chips # of extractions Chips' weight (g) Fat weight (g) Regular 3 20.043 6.745 3 20.187 6.438 3 20.198 7.451 Low fat 3 19.456 3.982 3 20.072 4.547 3 20.192 4.589 Mass percent is a method of expressing the concentration of a substance in a mixture or element in a compound. It is calculated as the mass of the component divided by the total mass of the mixture and then multiplied by 100 to get the percent. The formula is: mass % of component= (mass of component / total mass) x 100% 1) Reproduce the following table, and use the data to determine the mass % of fat in the chips for each trial. 2) Show one example calculation. Chips Trial % by mass of fat Regular 1 2 3 Lowfat 1 2 3Parts G and H. I thought the answers were 56.21 and 115.21 but it says they're wrong.Hi! I am having difficulty finishing and finding out the rest of tables in excel. I am trying to find the Calcium Carbonate in an Eggshell. I have put up the excel sheet I have done with the given data already inputed, I just need to find out the rest. Thank you so much!

