Chromel is an alloy composed of nickel, iron, and chromium. A 0.6418-g sample was dissolved and diluted to 250.0 mL. When a 50.00-mL aliquot of 0.05173 M EDTA was mixed with an equal volume of the diluted sample, all three ions were chelated, and a 5.27-mL back-titration with 0.06139 M copper(II) was required. The chromium in a second 50.0-mL aliquot was masked through the addition of hexamethylenetetramine; titration of the Fe and Ni required 35.81 mL of 0.05173 M EDTA. Iron and chromium were masked with pyrophosphate in a third 50.0-mL aliquot, and the nickel was titrated with 25.77 mL of the EDTA solution. Calculate the percentages of nickel, chromium, and iron in the alloy. Percentage of nickel = % Percentage of iron = 1% Percentage of chromium =
Chromel is an alloy composed of nickel, iron, and chromium. A 0.6418-g sample was dissolved and diluted to 250.0 mL. When a 50.00-mL aliquot of 0.05173 M EDTA was mixed with an equal volume of the diluted sample, all three ions were chelated, and a 5.27-mL back-titration with 0.06139 M copper(II) was required. The chromium in a second 50.0-mL aliquot was masked through the addition of hexamethylenetetramine; titration of the Fe and Ni required 35.81 mL of 0.05173 M EDTA. Iron and chromium were masked with pyrophosphate in a third 50.0-mL aliquot, and the nickel was titrated with 25.77 mL of the EDTA solution. Calculate the percentages of nickel, chromium, and iron in the alloy. Percentage of nickel = % Percentage of iron = 1% Percentage of chromium =
Chapter17: Complexation And Precipitation Reactions And Titrations
Section: Chapter Questions
Problem 17.31QAP
Related questions
Question
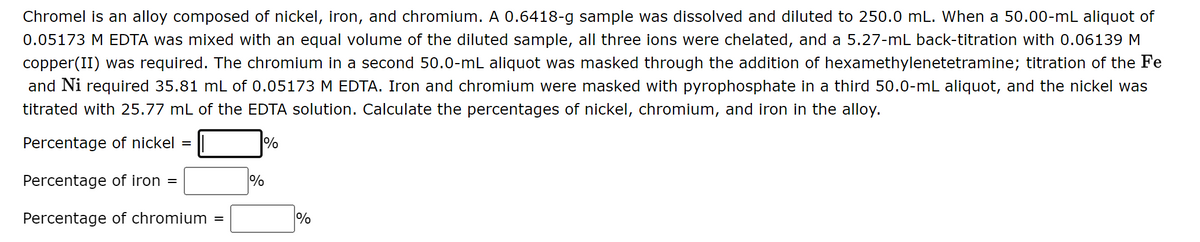
Transcribed Image Text:Chromel is an alloy composed of nickel, iron, and chromium. A 0.6418-g sample was dissolved and diluted to 250.0 mL. When a 50.00-mL aliquot of
0.05173 M EDTA was mixed with an equal volume of the diluted sample, all three ions were chelated, and a 5.27-mL back-titration with 0.06139 M
copper(II) was required. The chromium in a second 50.0-mL aliquot was masked through the addition of hexamethylenetetramine; titration of the Fe
and Ni required 35.81 mL of 0.05173 M EDTA. Iron and chromium were masked with pyrophosphate in a third 50.0-mL aliquot, and the nickel was
titrated with 25.77 mL of the EDTA solution. Calculate the percentages of nickel, chromium, and iron in the alloy.
Percentage of nickel =
%
Percentage of iron =
Percentage of chromium =
%
%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning