Chromium has an atomic mass of 51.9961 u and consists of four isotopes, 50 Cr, 2Cr, 53Cr, and Cr. The 2Cr isotope has a natural abundance of 83.79% and an atomic mass of 51.9405 u. The $Cr isotope has a natural abundance of 2.37% and an atomic mass of 53.9389 u. The natural abundances of the 5° Cr and 53 Cr isotopes exist in a ratio of 0.4579:1, and the 50 Cr isotope has an atomic mass of 49.9460 u. Determine the atomic mass of the 5Cr isotope. atomic mass of 53 Cr: u
Chromium has an atomic mass of 51.9961 u and consists of four isotopes, 50 Cr, 2Cr, 53Cr, and Cr. The 2Cr isotope has a natural abundance of 83.79% and an atomic mass of 51.9405 u. The $Cr isotope has a natural abundance of 2.37% and an atomic mass of 53.9389 u. The natural abundances of the 5° Cr and 53 Cr isotopes exist in a ratio of 0.4579:1, and the 50 Cr isotope has an atomic mass of 49.9460 u. Determine the atomic mass of the 5Cr isotope. atomic mass of 53 Cr: u
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section: Chapter Questions
Problem 20PS: Strontium has four stable isotopes. Strontium-84 has a very low natural abundance, but 86Sr, 87Sr,...
Related questions
Question
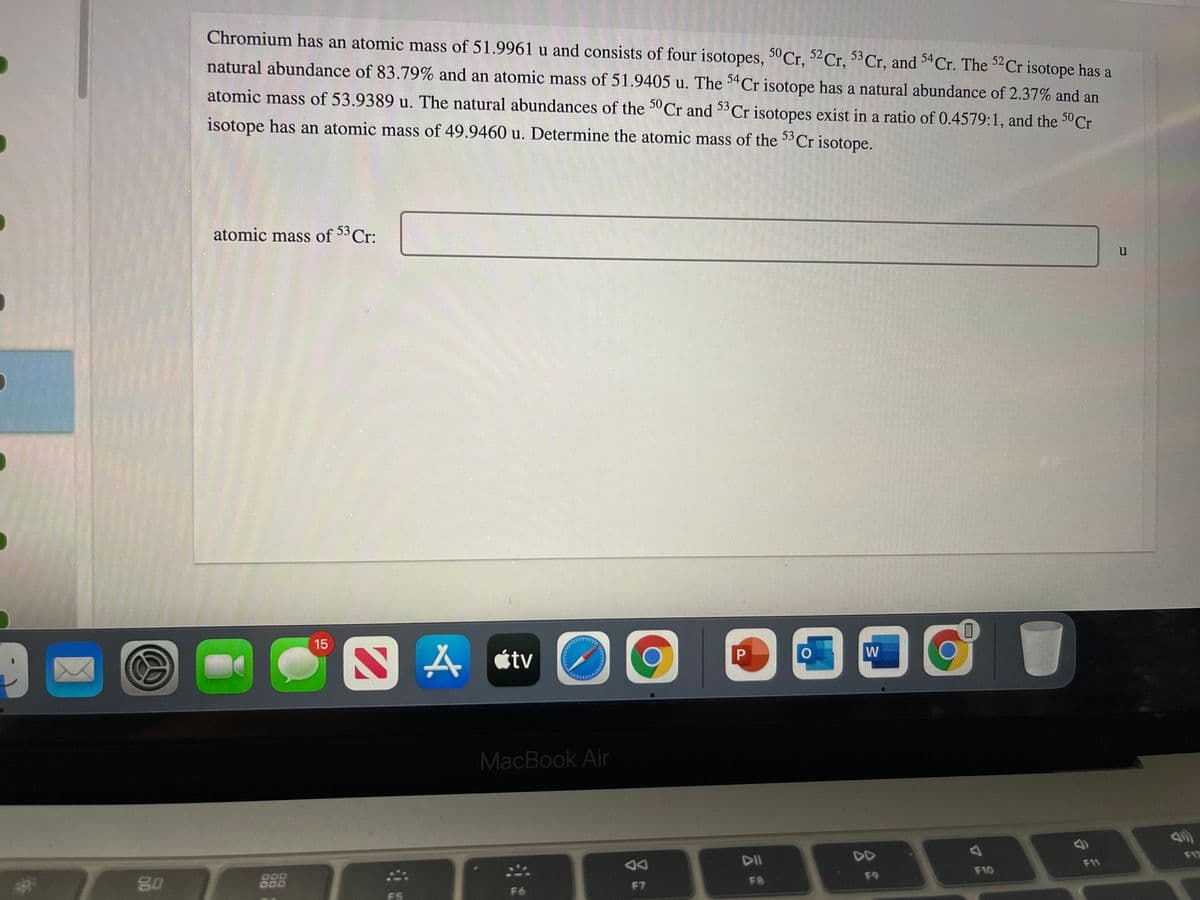
Transcribed Image Text:Chromium has an atomic mass of 51.9961 u and consists of four isotopes, 5° Cr, 52Cr, Cr, and 34Cr. The ²Cr isotope has a
natural abundance of 83.79% and an atomic mass of 51.9405 u. The 34Cr isotope has a natural abundance of 2.37% and an
atomic mass of 53.9389 u. The natural abundances of the 3°Cr and 33Cr isotopes exist in a ratio of 0.4579:1, and the 3°C.
isotope has an atomic mass of 49.9460 u. Determine the atomic mass of the 33Cr isotope.
atomic mass of 53 Cr:
u
15
A étv
W
MacBook Air
DII
DD
80
DO0
F12
F11
F7
FB
F9
F10
F5
F6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
