3. Let's try out the atomic mass equation. Suppose you have a mixture of two nitrogen isotopes. For every 1 Nitrogen-15 isotopes, there are 3 Nitrogen-14 isotopes. Determine the abundance of each isotope in this mixture. Predict the average atomic mass of this mixture. Hint: This is NOT the mixture of nitrogen found in nature. You can check your answer by clicking "My Mixture" under isotope mixture. Next, create the mixture described in the problem. 14.50 amu 14.00 amu 14.25 amu 14.75 amu
3. Let's try out the atomic mass equation. Suppose you have a mixture of two nitrogen isotopes. For every 1 Nitrogen-15 isotopes, there are 3 Nitrogen-14 isotopes. Determine the abundance of each isotope in this mixture. Predict the average atomic mass of this mixture. Hint: This is NOT the mixture of nitrogen found in nature. You can check your answer by clicking "My Mixture" under isotope mixture. Next, create the mixture described in the problem. 14.50 amu 14.00 amu 14.25 amu 14.75 amu
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter5: Stoichiometry
Section: Chapter Questions
Problem 23Q: Reference Section 5-2 to find the atomic masses of 12C and 13C, the relative abundance of 12C and...
Related questions
Question
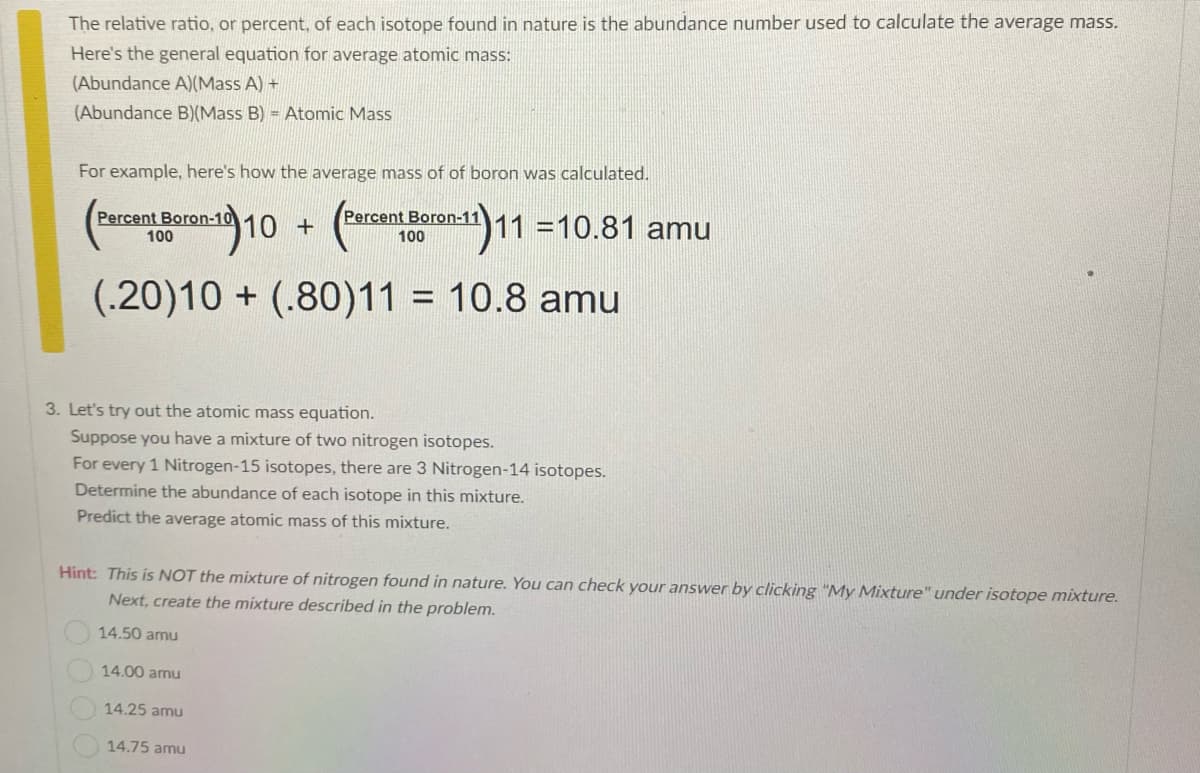
Transcribed Image Text:The relative ratio, or percent, of each isotope found in nature is the abundance number used to calculate the average mass.
Here's the general equation for average atomic mass:
(Abundance A)(Mass A) +
(Abundance B)(Mass B) = Atomic Mass
For example, here's how the average mass of of boron was calculated.
Percent Boron-10
Percent Boron-11
10 +
11 =10.81 amu
100
100
(.20)10 + (.80)11 = 10.8 amu
%D
3. Let's try out the atomic mass equation.
Suppose you have a mixture of two nitrogen isotopes.
For every 1 Nitrogen-15 isotopes, there are 3 Nitrogen-14 isotopes.
Determine the abundance of each isotope in this mixture.
Predict the average atomic mass of this mixture.
Hint: This is NOT the mixture of nitrogen found in nature. You can check your answer by clicking "My Mixture" under isotope mixture.
Next, create the mixture described in the problem.
14.50 amu
14.00 amu
14.25 amu
14.75 amu
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning