Classify the following reactions as synthesis, decomposition, single-displacement, or double-displacement reactions. Drag the items to their respective bins. • View Available Hint(s) Reset Help 2Fe + 6HC1 – 2FEC13 + 3H2 Na,CO3 + 2HCI 2NaCl + H2CO3 CaCO, + CaO + CO2 2NO - N2 + O2 CH12 + Br2 → CH12B12 Hg2(NO3)2 + 2NACI → 2NANO, + Hg,Cl2 2HCI - H2 + Cl2 8Fe + Sg → 8Fes Synthesis Decomposition Single-displacement Double-displacement
Classify the following reactions as synthesis, decomposition, single-displacement, or double-displacement reactions. Drag the items to their respective bins. • View Available Hint(s) Reset Help 2Fe + 6HC1 – 2FEC13 + 3H2 Na,CO3 + 2HCI 2NaCl + H2CO3 CaCO, + CaO + CO2 2NO - N2 + O2 CH12 + Br2 → CH12B12 Hg2(NO3)2 + 2NACI → 2NANO, + Hg,Cl2 2HCI - H2 + Cl2 8Fe + Sg → 8Fes Synthesis Decomposition Single-displacement Double-displacement
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section: Chapter Questions
Problem 36QRT
Related questions
Question
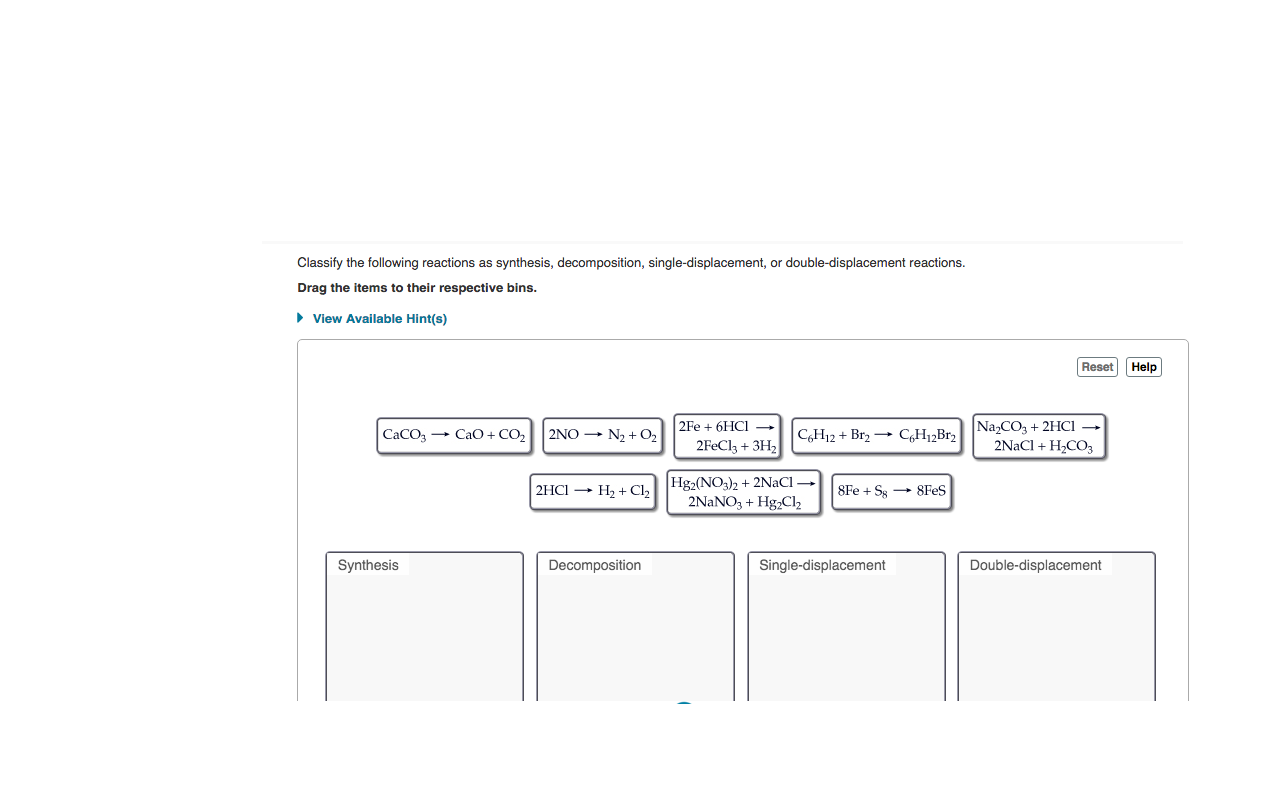
Transcribed Image Text:Classify the following reactions as synthesis, decomposition, single-displacement, or double-displacement reactions.
Drag the items to their respective bins.
• View Available Hint(s)
Reset Help
2Fe + 6HC1 –
2FEC13 + 3H2
Na,CO3 + 2HCI
2NaCl + H2CO3
CaCO, + CaO + CO2
2NO - N2 + O2
CH12 + Br2 → CH12B12
Hg2(NO3)2 + 2NACI →
2NANO, + Hg,Cl2
2HCI - H2 + Cl2
8Fe + Sg → 8Fes
Synthesis
Decomposition
Single-displacement
Double-displacement
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax