Colligative properties are those that depend on the number of solute particles. Because electrolytes dissociate into ions, the concentration of particles in the solution is greater than the formula-unit concentration of the solution. For example, if 1 mol of NazSO4 totally dissociates, 3 mol of ions are produced (2 mol of Nat ions and 1 mol of SO, ions). Thus, a colligative property such as osmotic pressure will be three times greater for a 1 M NazSO, solution than for a 1 M nonelectrolyte solution. Part A Assuming complete dissociation of the solute, how many grams of KNO, must be added to 275 mL of water to produce a solution that freezes at -14.5 °C? The freezing point for pure water is 0.0 °C and Kf is equal to 1.86 °C/m. Express your answer to three significant figures and include the appropriate units. > View Available Hint(s) However, complete dissociation of electrolytes does not always occur. The extent of dissociation the van't Hoff factor, i: expressed by O B) ? moles of particles in solution moles of solute dissolved 7.80 kg The equations for colligative properties can be written to include i. For example. AT = K - m-i Submit Previous Answers AT K m-i
Colligative properties are those that depend on the number of solute particles. Because electrolytes dissociate into ions, the concentration of particles in the solution is greater than the formula-unit concentration of the solution. For example, if 1 mol of NazSO4 totally dissociates, 3 mol of ions are produced (2 mol of Nat ions and 1 mol of SO, ions). Thus, a colligative property such as osmotic pressure will be three times greater for a 1 M NazSO, solution than for a 1 M nonelectrolyte solution. Part A Assuming complete dissociation of the solute, how many grams of KNO, must be added to 275 mL of water to produce a solution that freezes at -14.5 °C? The freezing point for pure water is 0.0 °C and Kf is equal to 1.86 °C/m. Express your answer to three significant figures and include the appropriate units. > View Available Hint(s) However, complete dissociation of electrolytes does not always occur. The extent of dissociation the van't Hoff factor, i: expressed by O B) ? moles of particles in solution moles of solute dissolved 7.80 kg The equations for colligative properties can be written to include i. For example. AT = K - m-i Submit Previous Answers AT K m-i
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter16: Solutions
Section: Chapter Questions
Problem 14E
Related questions
Question
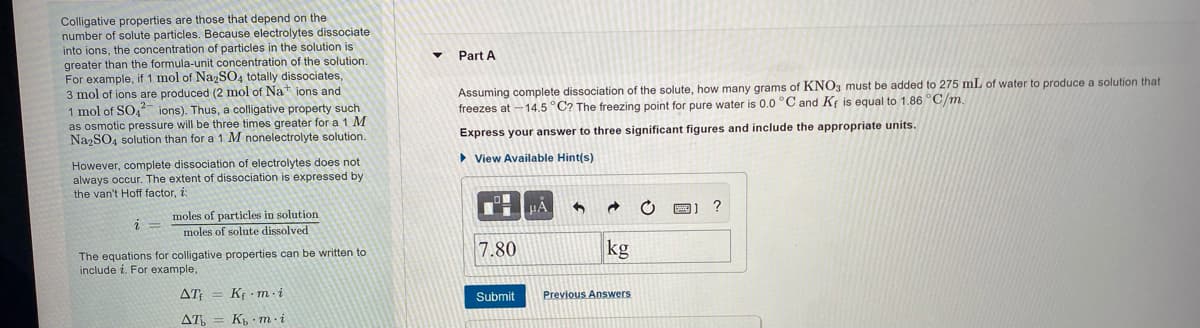
Transcribed Image Text:Colligative properties are those that depend on the
number of solute particles. Because electrolytes dissociate
into ions, the concentration of particles in the solution is
greater than the formula-unit concentration of the solution.
For example, if 1 mol of NazSO4 totally dissociates,
3 mol of ions are produced (2 mol of Nat ions and
1 mol of SO, ions). Thus, a colligative property such
as osmotic pressure will be three times greater for a 1 M
NazSO, solution than for a 1 M nonelectrolyte solution.
Part A
Assuming complete dissociation of the solute, how many grams of KNO3 must be added to 275 mL of water to produce a solution that
freezes at - 14.5 °C? The freezing point for pure water is 0.0 °C and Kf is equal to 1.86 °C/m.
Express your answer to three significant figures and include the appropriate units.
• View Available Hint(s)
However, complete dissociation of electrolytes does not
always occur. The extent
the van't Hoff factor, i:
dissociation is expressed by
HA
moles of particles in solution
moles of solute dissolved
7.80
kg
The equations for colligative properties can be written to
include i. For example,
Δ
K - m-i
Submit
Previous Answers
AT,
K m i
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning