COLOR THEME 13. Several JASON learning ambassadors for Aldine ISD recorded a demonstration for the students. They mixed two chemicals together and produced a 'golden rain'. The equation for the reaction is: KI + Pb(NO3)2 KNO3 + PbI₂ 4 The golden substance (image below) was found to be insoluble in water. Which of the following compounds is the precipitate? BROVOUS
COLOR THEME 13. Several JASON learning ambassadors for Aldine ISD recorded a demonstration for the students. They mixed two chemicals together and produced a 'golden rain'. The equation for the reaction is: KI + Pb(NO3)2 KNO3 + PbI₂ 4 The golden substance (image below) was found to be insoluble in water. Which of the following compounds is the precipitate? BROVOUS
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.82PAE
Related questions
Question
Transcribed Image Text:S
Eduphoria
→
CX
Science Chemistry 4th NW DCA 22-23
|
W
COLOR THEME
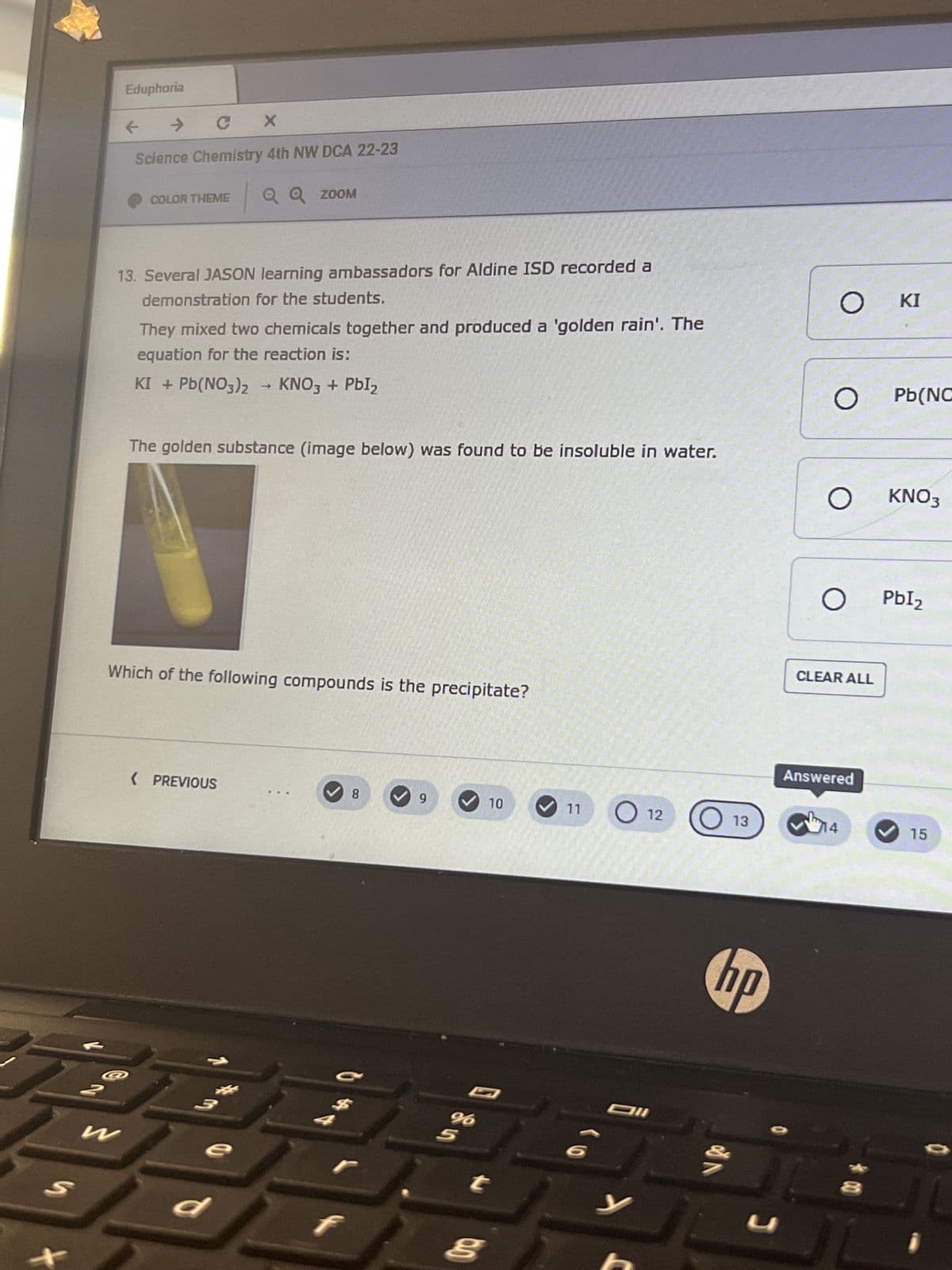
13. Several JASON learning ambassadors for Aldine ISD recorded a
demonstration for the students.
They mixed two chemicals together and produced a 'golden rain'. The
equation for the reaction is:
KI + Pb(NO3)2 → KNO3 + PbI₂
The golden substance (image below) was found to be insoluble in water.
Which of the following compounds is the precipitate?
Q Q ZOOM
< PREVIOUS
3
e
d
8
C
9
10
t
Oro
11
12
Oll
y
O
13
hp
U
O
O
O KNO3
CLEAR ALL
ΚΙ
O PbI₂
Answered
14
Pb(NC
15
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning