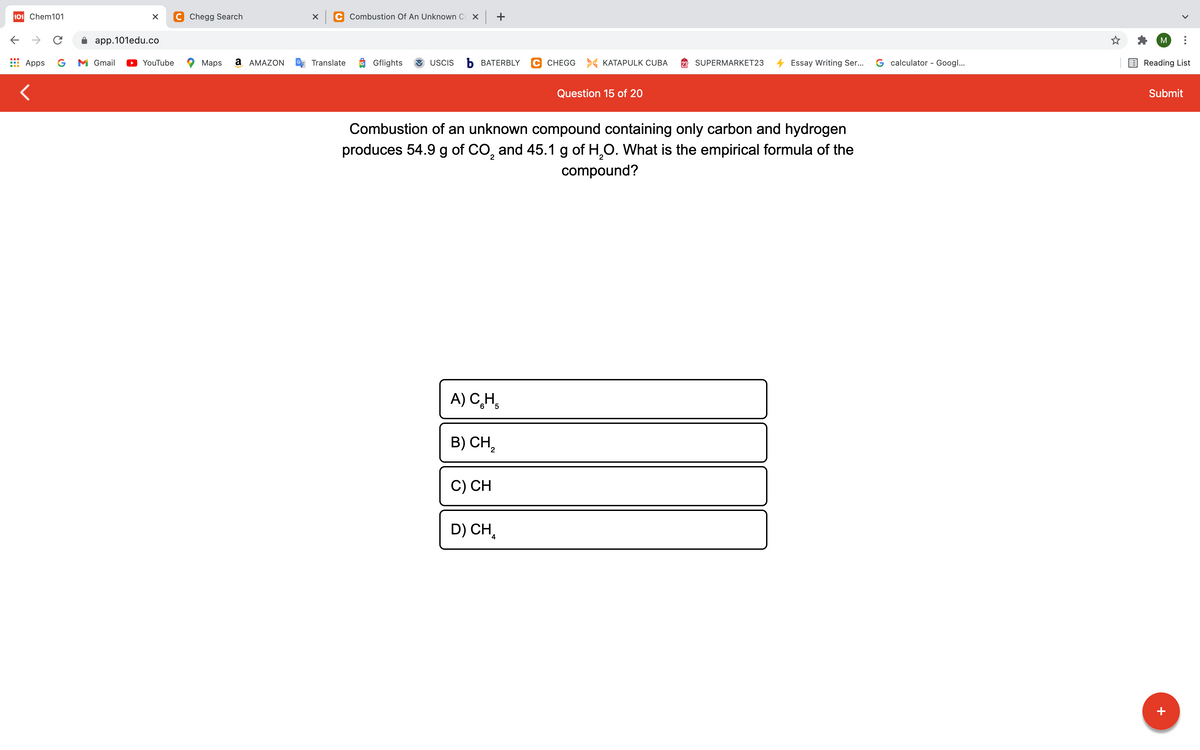
Combustion of an unknown compound containing only carbon and hydrogen produces 54.9 g of co, and 45.1 g of H,O. What is the empirical formula of the compound? A) C,H, B) CH, C) CH D) CH,
Combustion of an unknown compound containing only carbon and hydrogen produces 54.9 g of co, and 45.1 g of H,O. What is the empirical formula of the compound? A) C,H, B) CH, C) CH D) CH,
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 4E
Related questions
Question
Transcribed Image Text:101 Chem101
C Chegg Search
C Combustion Of An Unknown C X +
app.101edu.co
M
Apps
G
M Gmail
YouTube
Maps
a AMAZON
Translate
Gflights
USCIS
Ь ВАТERBLY
C CHEGG KATAPULK CUBA
SUPERMARKET23
Essay Writing Ser...
G calculator - Googl...
Reading List
Question 15 of 20
Submit
Combustion of an unknown compound containing only carbon and hydrogen
produces 54.9 g of CO, and 45.1 g of H,O. What is the empirical formula of the
compound?
A) C,H,
B) CH,
C) CH
D) CH,
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning