Compare the value you calculated with the actual value (-601.8 kJ/mol). Show your determination of percent error. Use the standard equation for this: actual value - experimental value %error = x 100% |actual value|
Compare the value you calculated with the actual value (-601.8 kJ/mol). Show your determination of percent error. Use the standard equation for this: actual value - experimental value %error = x 100% |actual value|
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.94QP
Related questions
Question
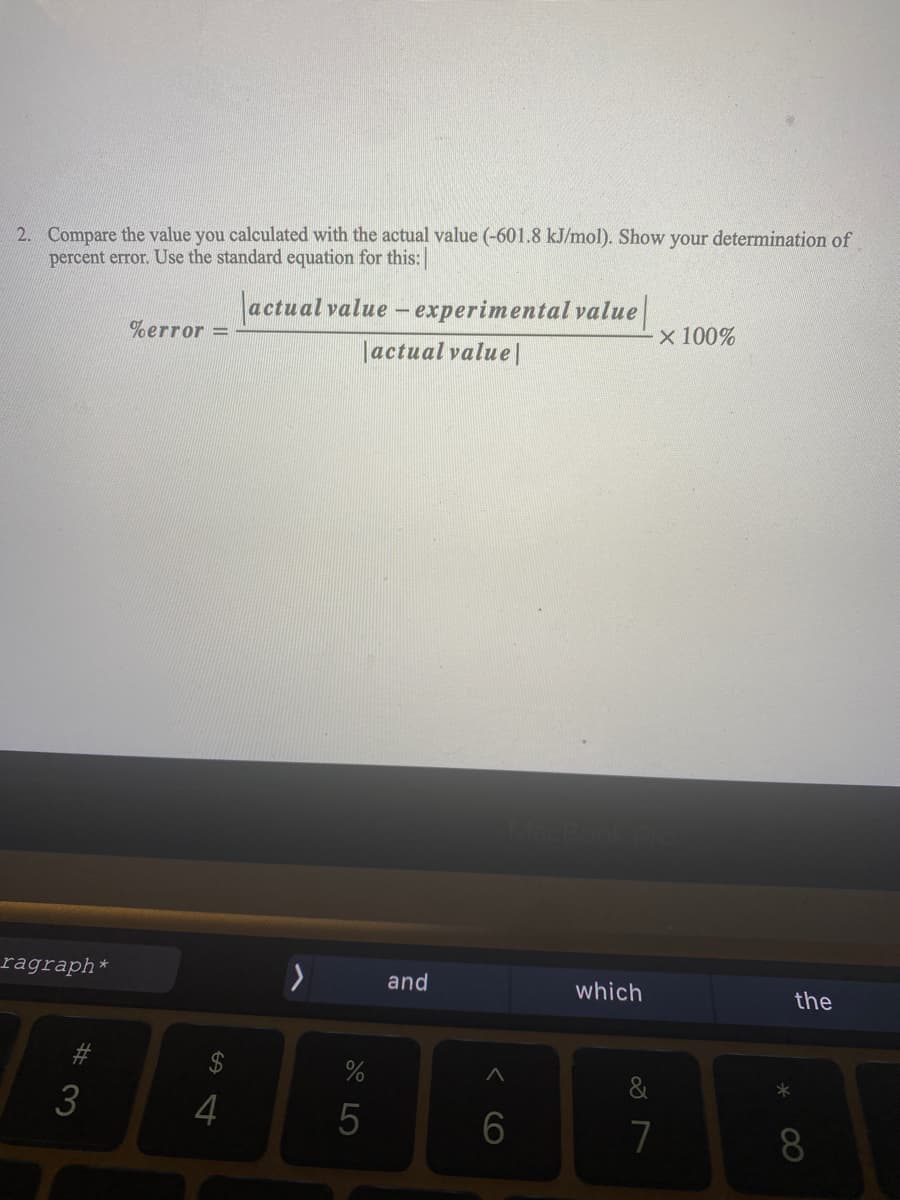
Transcribed Image Text:2. Compare the value you calculated with the actual value (-601.8 kJ/mol). Show your determination of
percent error. Use the standard equation for this:
actual value - experimental value
%error =
x 100%
|actual value|
ragraph*
and
which
the
&
3
4
7
8
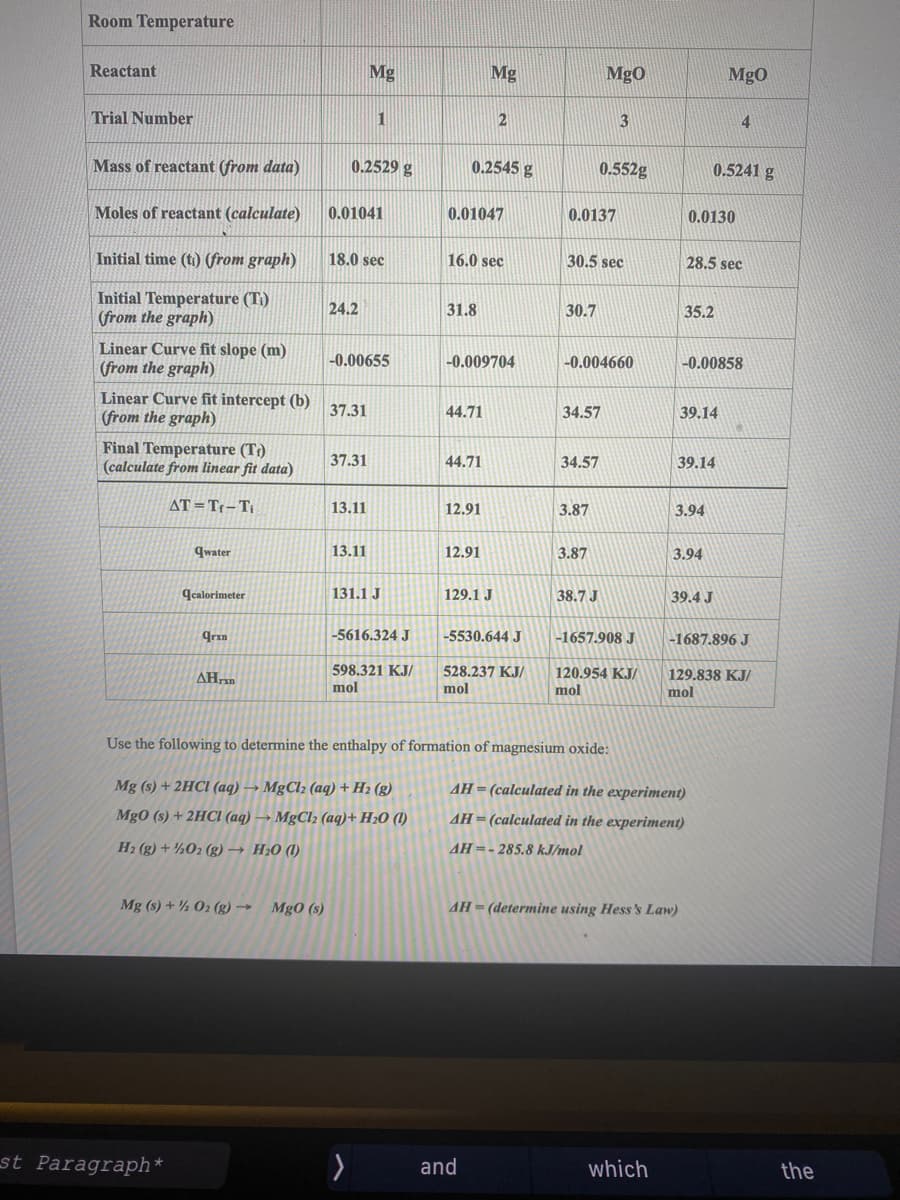
Transcribed Image Text:Room Temperature
Reactant
Mg
Mg
MgO
MgO
Trial Number
1
2
3
4
Mass of reactant (from data)
0.2529 g
0.2545 g
0.552g
0.5241 g
Moles of reactant (calculate)
0.01041
0.01047
0.0137
0.0130
Initial time (ti) (from graph)
18.0 sec
16.0 sec
30.5 sec
28.5 sec
Initial Temperature (Ti)
(from the graph)
24.2
31.8
30.7
35.2
Linear Curve fit slope (m)
(from the graph)
-0.00655
-0.009704
-0.004660
-0.00858
Linear Curve fit intercept (b)
(from the graph)
37.31
44.71
34.57
39.14
Final Temperature (T?)
(calculate from linear fit data)
37.31
44.71
34.57
39.14
AT =Tr-Ti
13.11
12.91
3.87
3.94
qwater
13.11
12,91
3.87
3.94
qcalorimeter
131.1 J
129.1 J
38.7 J
39.4 J
qrxn
-5616.324 J
-5530.644 J
-1657.908 J
-1687.896 J
598.321 KJ/
528.237 KJ/
mol
120.954 KJ/
mol
129.838 KJ/
mol
ΔΗ
mol
Use the following to determine the enthalpy of formation of magnesium oxide:
Mg (s) + 2HCI (aq) → MgCl2 (aq) + H2 (g)
AH= (calculated in the experiment)
Mg0 (s) + 2HCI (aq) → MgCl2 (aq)+ H20 (1)
AH = (calculated in the experiment)
H2 (g) + ½02 (g) → H20 (1)
AH=- 285.8 kJ/mol
Mg (s) + ½ 02 (g) →
Mg0 (s)
AH = (determine using Hess's Law)
st Paragraph*
and
which
the
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning