Compound 5 (shown below) displays intriguing reactivity features at distinct pHs. We will explore several examples. OH pH 13 OH ОН О LIOH 7 8 H20 ОН О ОН ОН pH 7 + CO2 HO ОН ОН ÓH Ö 10 (a) When 5 and 6 are treated with LIOH and the pH is further adjusted to highly basic (pH 13–14), products 7 and 8 are predominantly formed. Propose a reasonable mechanism for this reaction. OH OH LIOH рH 13 H H2O ÓH O ÓH Ö 7 Hints: a somewhat challenging pathway; start with Aldol chemistry and recall the extreme pH! You may be able to “take advantage" of intramolecular H transfer. (b) When 5 and 6 are treated with LIOH and the pH is further adjusted close to neutral (pH 6–8), products 9 and 10 (in addition to CO2) are predominantly formed. Propose a reasonable mechanism for this reaction. OH ОН ОН LIOH pH 7 H H20 но + CO2 О ОН ОН OH O 6 10
Compound 5 (shown below) displays intriguing reactivity features at distinct pHs. We will explore several examples. OH pH 13 OH ОН О LIOH 7 8 H20 ОН О ОН ОН pH 7 + CO2 HO ОН ОН ÓH Ö 10 (a) When 5 and 6 are treated with LIOH and the pH is further adjusted to highly basic (pH 13–14), products 7 and 8 are predominantly formed. Propose a reasonable mechanism for this reaction. OH OH LIOH рH 13 H H2O ÓH O ÓH Ö 7 Hints: a somewhat challenging pathway; start with Aldol chemistry and recall the extreme pH! You may be able to “take advantage" of intramolecular H transfer. (b) When 5 and 6 are treated with LIOH and the pH is further adjusted close to neutral (pH 6–8), products 9 and 10 (in addition to CO2) are predominantly formed. Propose a reasonable mechanism for this reaction. OH ОН ОН LIOH pH 7 H H20 но + CO2 О ОН ОН OH O 6 10
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter21: Benzene And The Concept Of Aromaticity
Section: Chapter Questions
Problem 21.26P: Compound I (C11H14O2) is insoluble in water, aqueous acid, and aqueous NaHCO3, but dissolves readily...
Related questions
Question
Please provide mechanisms for these reactions
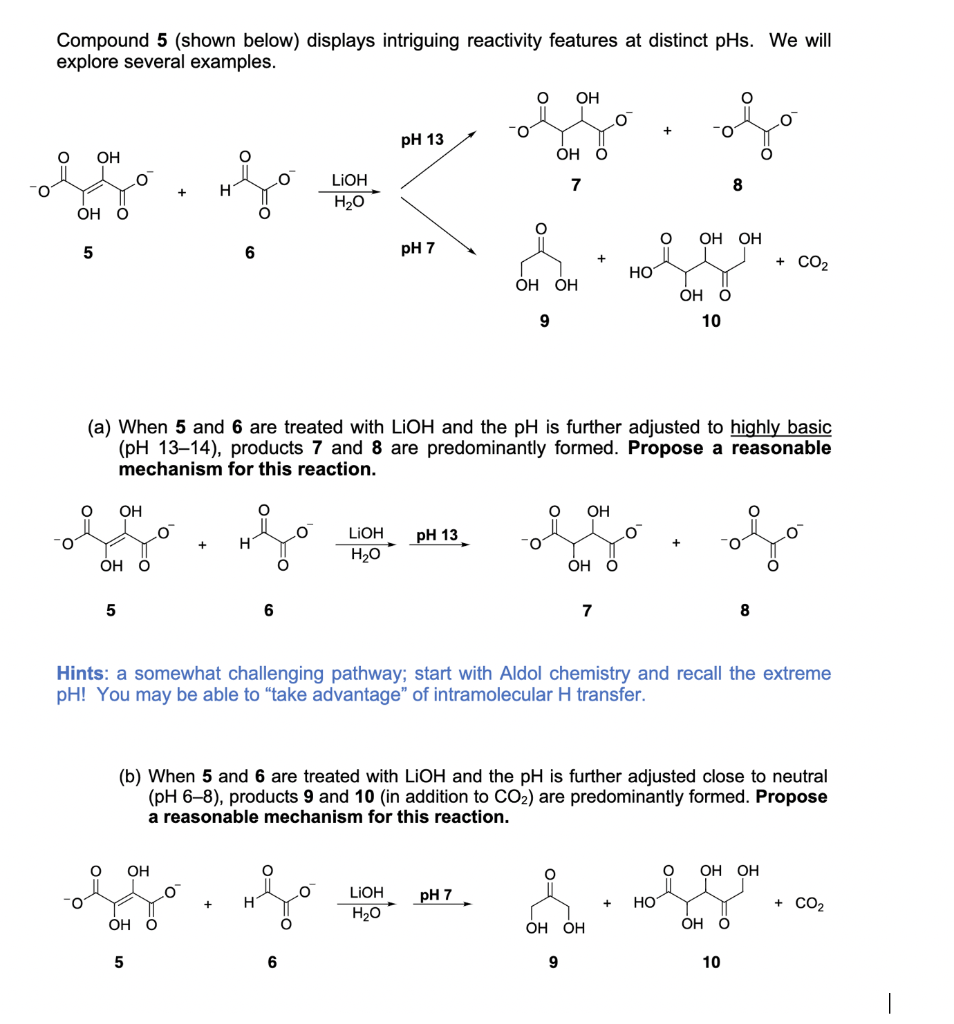
Transcribed Image Text:Compound 5 (shown below) displays intriguing reactivity features at distinct pHs. We will
explore several examples.
OH
O.
рH 13
OH
ОН О
LIOH
7
8
H2O
ОН О
ОН ОН
5
6
pH 7
+ CO2
HO
ОН ОН
ОН О
10
(a) When 5 and 6 are treated with LIOH and the pH is further adjusted to highly basic
(pH 13–14), products 7 and 8 are predominantly formed. Propose a reasonable
mechanism for this reaction.
OH
OH
LIOH
pH 13
O.
H2O
Он О
ОН О
7
8
Hints: a somewhat challenging pathway; start with Aldol chemistry and recall the extreme
pH! You may be able to "take advantage" of intramolecular H transfer.
(b) When 5 and 6 are treated with LIOH and the pH is further adjusted close to neutral
(pH 6-8), products 9 and 10 (in addition to CO2) are predominantly formed. Propose
a reasonable mechanism for this reaction.
OH
ОН ОН
LIOH
pH 7
HO
+ CO2
H2O
ÓH Ö
ОН ОН
ОН О
5
10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
