Compound 67 is a high-boiling liquid (boiling point 180° C) that reacts with I, in aqueous base to give a yellow precipitate of CHL, (the jodoform test). The compound is readily prepared by the condensation of two moles of ethyl acetate in the presence of ethanol/ ethoxide anion. The Mass, IR, and 'H NMR spectra, along with 13C NMR data, are given below. Elemental Analysis: C, 55.37; H, 7.74; O, 36.88. Mass Spectrum 143 88 130 115 60 70 100 110 120 130 140 150 160 170 180 190 200 30 40 50 80 90 m/e Infrared Spectrum Wave Number, cm -1 1500 1300 1200 1100 1000 800 700 4000 3000 2500 2000 006 Absorbance Intensity
Compound 67 is a high-boiling liquid (boiling point 180° C) that reacts with I, in aqueous base to give a yellow precipitate of CHL, (the jodoform test). The compound is readily prepared by the condensation of two moles of ethyl acetate in the presence of ethanol/ ethoxide anion. The Mass, IR, and 'H NMR spectra, along with 13C NMR data, are given below. Elemental Analysis: C, 55.37; H, 7.74; O, 36.88. Mass Spectrum 143 88 130 115 60 70 100 110 120 130 140 150 160 170 180 190 200 30 40 50 80 90 m/e Infrared Spectrum Wave Number, cm -1 1500 1300 1200 1100 1000 800 700 4000 3000 2500 2000 006 Absorbance Intensity
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter21: Benzene And The Concept Of Aromaticity
Section: Chapter Questions
Problem 21.26P: Compound I (C11H14O2) is insoluble in water, aqueous acid, and aqueous NaHCO3, but dissolves readily...
Related questions
Question
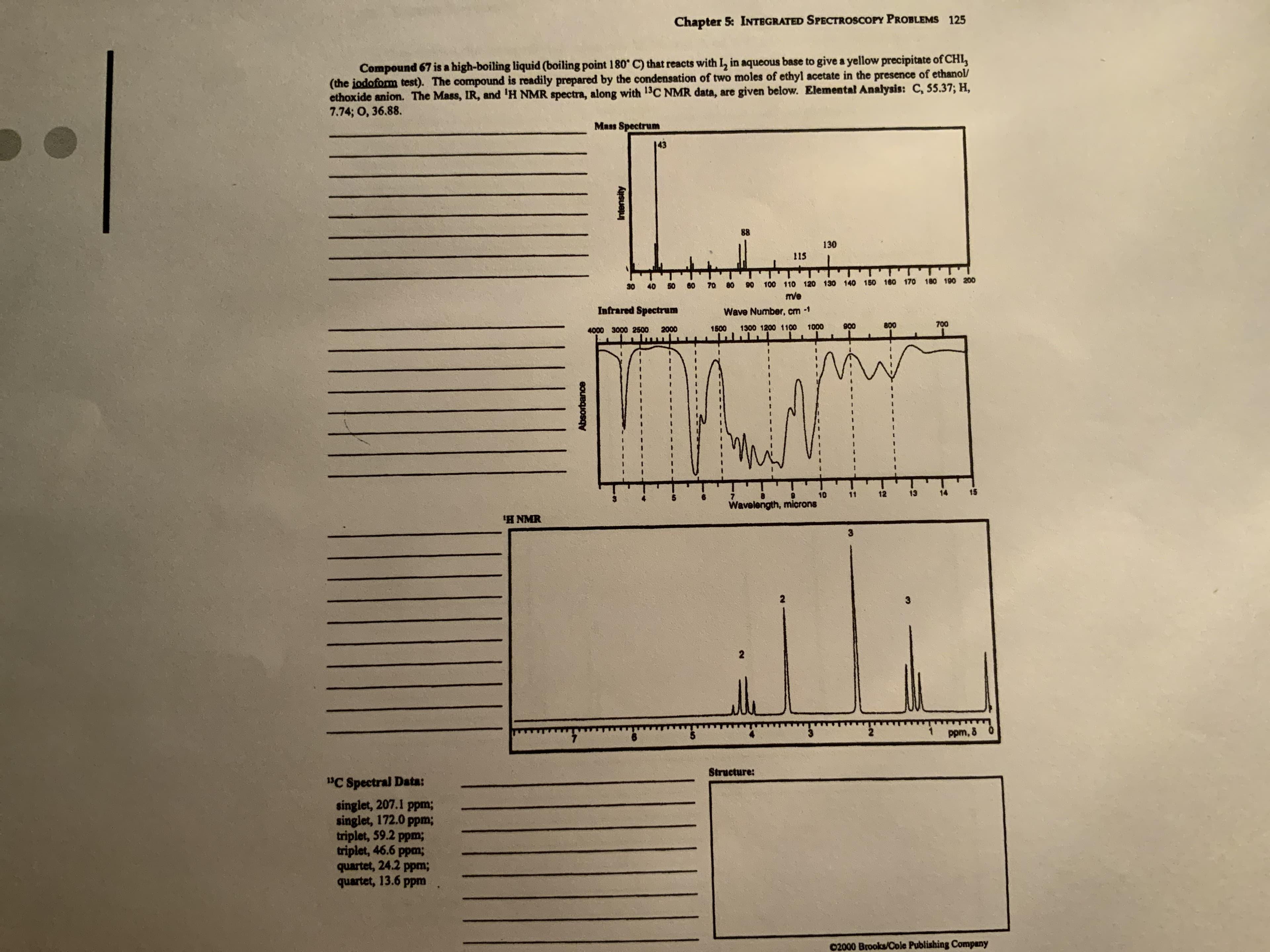
Transcribed Image Text:Compound 67 is a high-boiling liquid (boiling point 180° C) that reacts with I, in aqueous base to give a yellow precipitate of CHL,
(the jodoform test). The compound is readily prepared by the condensation of two moles of ethyl acetate in the presence of ethanol/
ethoxide anion. The Mass, IR, and 'H NMR spectra, along with 13C NMR data, are given below. Elemental Analysis: C, 55.37; H,
7.74; O, 36.88.
Mass Spectrum
143
88
130
115
60
70
100 110 120 130 140 150 160 170 180 190 200
30
40
50
80
90
m/e
Infrared Spectrum
Wave Number, cm -1
1500
1300 1200 1100
1000
800
700
4000 3000 2500
2000
006
Absorbance
Intensity
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole