If anhydrous platinum(II) bromide is suspended in chloroform and treated with o-styryldimethyl arsine (o-S), three compounds, A, B and C can be isolated. ALL compounds are monomeric and non-conducting in nitro- benzene. Formulae and infrared data are tabulated below: CH=CH2 Üc=c/ cm-1 o-S 1630 As (CH3)2 A PtBr,(o-S), 1630 B PtBr,(o-S)2 1627 C PtBr, Co-S) 1488 Addition of an excess of bromine to A or B gave an orange complex, D, PtBr, (o-S), in which the bands at 1630/1627 cm- were absent, but new bands had appeared at 594 and 572 cm-. Suggest structures for A, B, C, and D.
If anhydrous platinum(II) bromide is suspended in chloroform and treated with o-styryldimethyl arsine (o-S), three compounds, A, B and C can be isolated. ALL compounds are monomeric and non-conducting in nitro- benzene. Formulae and infrared data are tabulated below: CH=CH2 Üc=c/ cm-1 o-S 1630 As (CH3)2 A PtBr,(o-S), 1630 B PtBr,(o-S)2 1627 C PtBr, Co-S) 1488 Addition of an excess of bromine to A or B gave an orange complex, D, PtBr, (o-S), in which the bands at 1630/1627 cm- were absent, but new bands had appeared at 594 and 572 cm-. Suggest structures for A, B, C, and D.
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.17QAP
Related questions
Question
4
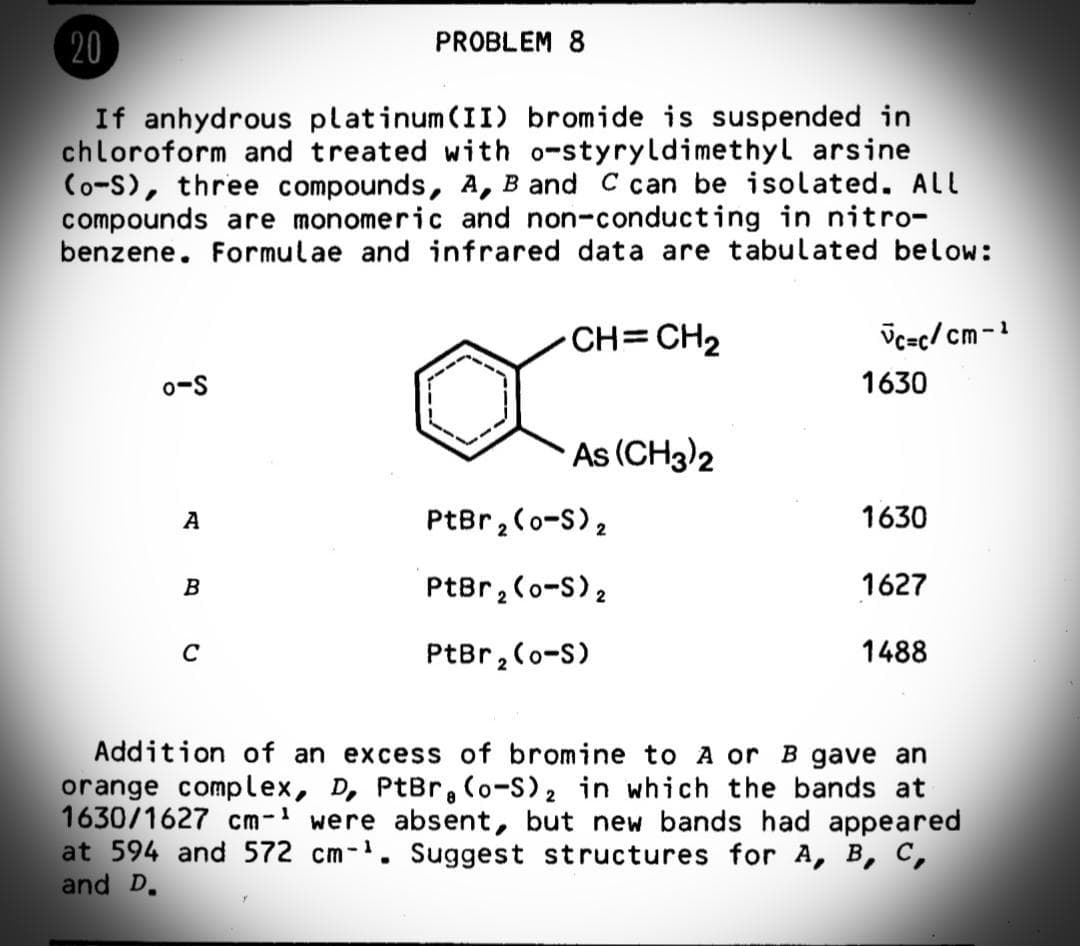
Transcribed Image Text:20
PROBLEM 8
If anhydrous platinum(II) bromide is suspended in
chloroform and treated with o-styryldimethyl arsine
(o-S), three compounds, A, B and C can be isolated. ALL
compounds are monomeric and non-conducting in nitro-
benzene. Formulae and infrared data are tabulated below:
CH=CH2
Vc=c/ cm-1
o-S
1630
As (CH3)2
PtBr, (o-S)2
1630
A
PtBr, (o-S)2
1627
B
C
PtBr, (o-S)
1488
Addition of an excess of bromine to A or B gave an
orange complex, D, PtBr, (o-S), in which the bands at
1630/1627 cm- were absent, but new bands had appeared
at 594 and 572 cm-. Suggest structures for A, B, C,
and D.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
