Compound a Compound b Compound c CH3COH CH3CCH2ČOCH2CH3 CH3CCH2CCH3 ethyl acetoacetate pkg = 11 pentane-2,4-dione pk, = 9 acetic acid pK, = 4.76 Which of the compounds above are strong enough acids to react almost completely with a hydroxide ion (pK, of H2O = 15.74) or with a bicarbonate ion (pK, of H2CO3 = 6.37)? Enter your answers as an alphabetized string, i.e. abc, not cba; enter none if none of the compounds are strong enough acids. Hydroxide ion: Bicarbonate ion:
Compound a Compound b Compound c CH3COH CH3CCH2ČOCH2CH3 CH3CCH2CCH3 ethyl acetoacetate pkg = 11 pentane-2,4-dione pk, = 9 acetic acid pK, = 4.76 Which of the compounds above are strong enough acids to react almost completely with a hydroxide ion (pK, of H2O = 15.74) or with a bicarbonate ion (pK, of H2CO3 = 6.37)? Enter your answers as an alphabetized string, i.e. abc, not cba; enter none if none of the compounds are strong enough acids. Hydroxide ion: Bicarbonate ion:
Chapter27: Biomolecules: Lipids
Section27.SE: Something Extra
Problem 47AP: Cembrene, C20H32, is a diterpenoid hydrocarbon isolated from pine resin. Cembrene has a UV...
Related questions
Question
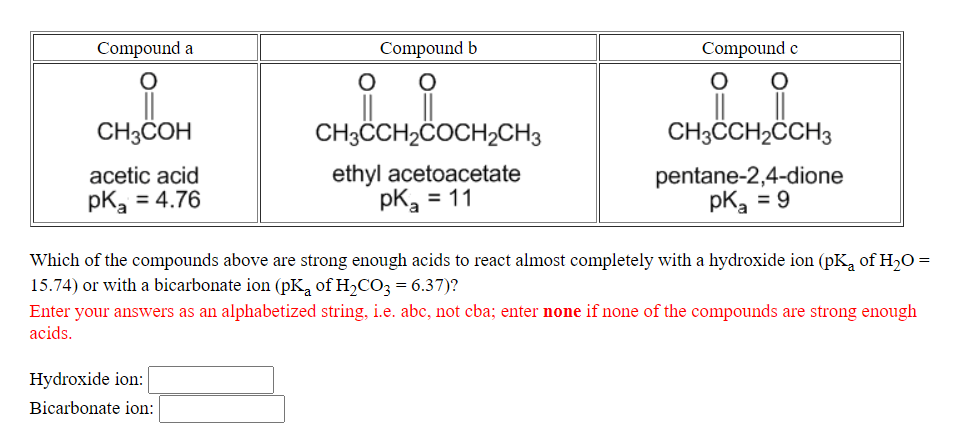
Transcribed Image Text:Compound a
Compound b
Compound e
CH3COH
CH3CH2ČOCH2CH3
CH3CH2CCH3
ethyl acetoacetate
pK, = 11
pentane-2,4-dione
pk, = 9
acetic acid
pk, = 4.76
Which of the compounds above are strong enough acids to react almost completely with a hydroxide ion (pK, of H20 =
15.74) or with a bicarbonate ion (pK, of H,CO3 = 6.37)?
Enter your answers as an alphabetized string, i.e. abc, not cba; enter none if none of the compounds are strong enough
acids.
Hydroxide ion:
Bicarbonate ion:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning