Fumaric acid (select) O than maleic acid, giving it a higher melting point. Maleic acid is (select) O than fumaric acid, giving it greater H2O solubility. In maleic acid, (select) O stabilizes the conjugate base after one H is removed, making maleic acid more acidic than fumaric acid. When removing the second H, pK2 is lower for fumaric acid than maleic acid because (select)
Fumaric acid (select) O than maleic acid, giving it a higher melting point. Maleic acid is (select) O than fumaric acid, giving it greater H2O solubility. In maleic acid, (select) O stabilizes the conjugate base after one H is removed, making maleic acid more acidic than fumaric acid. When removing the second H, pK2 is lower for fumaric acid than maleic acid because (select)
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter2: Alkanes And Cycloalkanes
Section: Chapter Questions
Problem 2.64P
Related questions
Question
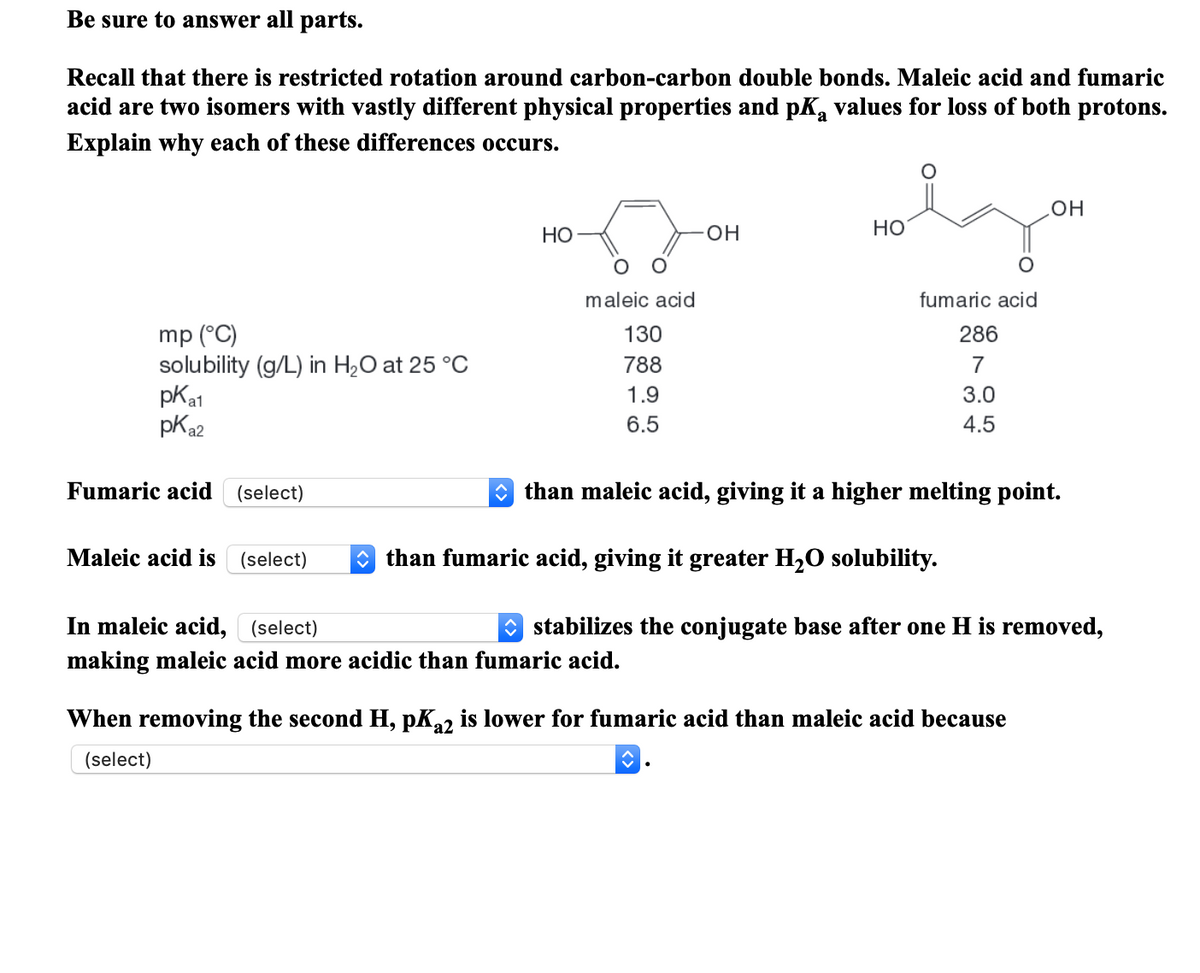
Transcribed Image Text:Be sure to answer all parts.
Recall that there is restricted rotation around carbon-carbon double bonds. Maleic acid and fumaric
acid are two isomers with vastly different physical properties and pK, values for loss of both protons.
Explain why each of these differences occurs.
он
Но
HO-
Но
maleic acid
fumaric acid
mp (°C)
solubility (g/L) in H2O at 25 °C
pKa1
pKa2
130
286
788
7
1.9
3.0
6.5
4.5
Fumaric acid
(select)
O than maleic acid, giving it a higher melting point.
Maleic acid is
(select)
O than fumaric acid, giving it greater H2O solubility.
In maleic acid, (select)
stabilizes the conjugate base after one H is removed,
making maleic acid more acidic than fumaric acid.
When removing the second H, pK22 is lower for fumaric acid than maleic acid because
(select)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning