Compound Naphthalene, C10H8 Carbon tetrachloride, CCI4 Acetone, CH3COCH3 Benzoic acid, C,H;COOH Boiling point (°C) AHvap(kJ/mol) 218 51.5 76 31.8 56 31.8 249 68.2 a) Naphthalene has a higher boiling point than carbon tetrachloride. b) Naphthalene has a lower vaporization energy than benzoic acid.
Compound Naphthalene, C10H8 Carbon tetrachloride, CCI4 Acetone, CH3COCH3 Benzoic acid, C,H;COOH Boiling point (°C) AHvap(kJ/mol) 218 51.5 76 31.8 56 31.8 249 68.2 a) Naphthalene has a higher boiling point than carbon tetrachloride. b) Naphthalene has a lower vaporization energy than benzoic acid.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter11: States Of Matter; Liquids And Solids
Section: Chapter Questions
Problem 11.49QP: Chloroform, CHCl3, a volatile liquid, was once used as an anesthetic but has been replaced by safer...
Related questions
Question
The Table below shows the boiling points and the energy required to evaporate various substances, use what we learned in chapter 3.7 regarding to intermolecular force to explain:
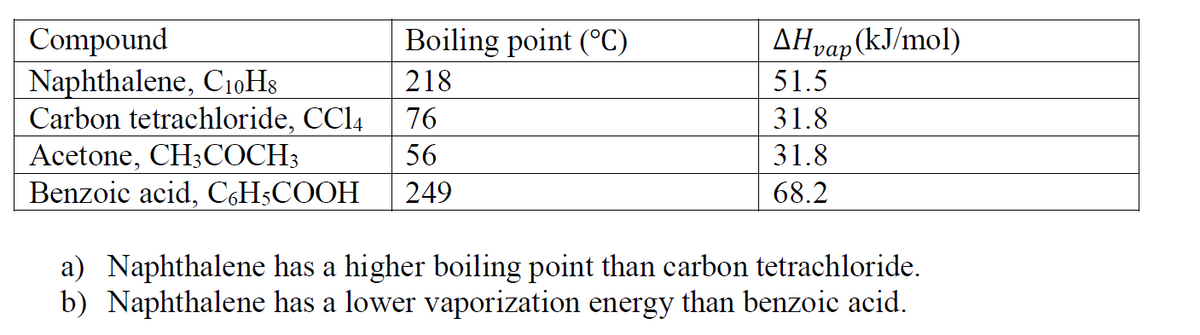
Transcribed Image Text:Compound
Naphthalene, C10H8
Carbon tetrachloride, CCI4
Acetone, CH;COCH3
Benzoic acid, C,H5COOH
Boiling point (°C)
AHvap (kJ/mol)
218
51.5
76
31.8
56
31.8
249
68.2
a) Naphthalene has a higher boiling point than carbon tetrachloride.
b) Naphthalene has a lower vaporization energy than benzoic acid.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning