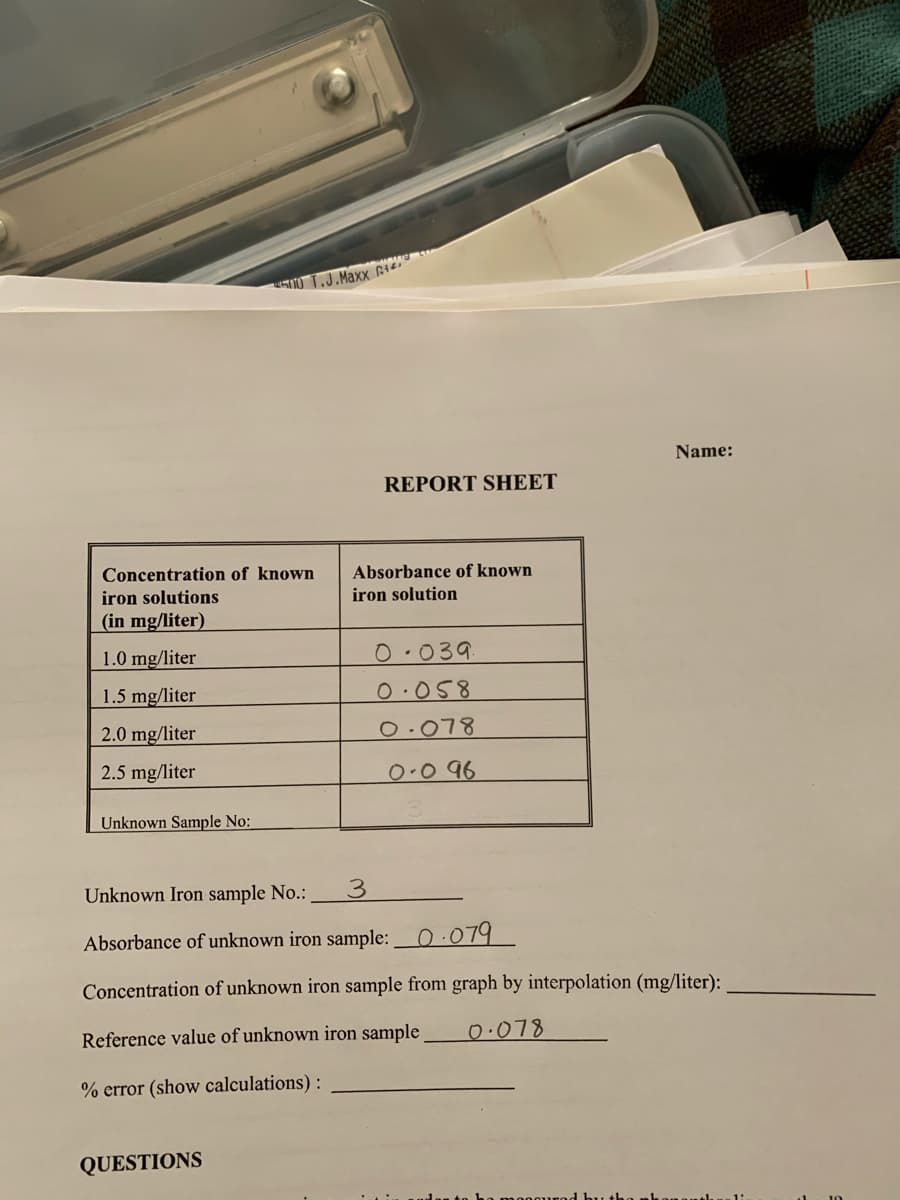
Concentration of known Absorbance of known iron solutions iron solution (in mg/liter) 1.0 mg/liter 0.039 1.5 mg/liter 0.058 2.0 mg/liter 0.078 2.5 mg/liter 0.0 96 Unknown Sample No:
Concentration of known Absorbance of known iron solutions iron solution (in mg/liter) 1.0 mg/liter 0.039 1.5 mg/liter 0.058 2.0 mg/liter 0.078 2.5 mg/liter 0.0 96 Unknown Sample No:
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.26QAP
Related questions
Question
I need help with this question
Transcribed Image Text:O T.J.Maxx Rie
Name:
REPORT SHEET
Concentration of known
Absorbance of known
iron solutions
iron solution
(in mg/liter)
1.0 mg/liter
0.039
1.5 mg/liter
0.058
2.0 mg/liter
0.078
2.5 mg/liter
0.0 96
Unknown Sample No:
Unknown Iron sample No.:
3.
Absorbance of unknown iron sample:O·079
Concentration of unknown iron sample from graph by interpolation (mg/liter):
Reference value of unknown iron sample
0.078
% error (show calculations) :
QUESTIONS

Transcribed Image Text:1:06
ul 5GE
Iron in Water Online La...
PROCEDURE:
1.
Obtain the standard iron solution whose concentration is 10 mg/liter. Fill a clean buret
with this standard iron solution. (Note: Be sure that you've rinsed the buret with 2 or 3 ml
of this iron solution before completely filling it.)
2.
Obtain four 100 ml volumetric flasks. Label these flasks as follows: 1.0 mg/liter, 1.5
mg/liter, 2.0 mg/liter, and 2.5 mg/liter.
3.
Into each flask, buret the proper amount of standard iron solution in order to prepare the
concentration written on each flask. Proceed as shown below,
Flask no.
Concentration
Milliliter of iron solution
to put into each flask
1
1.0 mg/liter
10 ml
1.5 mg/liter
15 ml
2.0 mg/liter
| 2.5 mg/liter
3
20 ml
4
25 ml
4.
Fill each flask with sufficient water to bring the total volume to 100 ml. The 100 ml mark
is noted on the neck of the volumetric flask. These four flasks now contain iron solutions
whose concentrations are 1.0 mg/liter, 1.5 mg/liter, 2.0 mg/liter, and 2.5 mg/liter.
5.
Using a 25-ml graduated cylinder, measure 25 ml of each standard and place it into a
100-ml beaker. (Note: Label these beakers so that you know which beaker contains a
particular iron solution.)
6.
Also place 25 ml of one Unknown solution into a 100-ml beaker.
Add the contents of one FerroVer powder pillow to cach beaker (standard and unknown),
and stir each solution. If iron is present, an orange-red color develops.
7.
8.
Let each solution stand for two minutes after adding the powder pillow. Then within ten
minutes, determine the absorbance of each solution.
9.
Use colorimeter to determine the absorbency of each solution. (Your instructor will
demonstrate how to operate the colorimeter).
10.
Construct a graph (use attached graph sheet). Plot absorbance (on y axis) versus
concentration (on x axis) for each of the known iron samples. From this graph and the
absorbance of your unknown sample, determine the concentration of iron in your
unknown sample.
Name:
Dashboard
Calendar
To Do
Notifications
Inbox
因
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you







Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT