Consider 1.50 mols of a substance in thermal equilibrium at a temperature of 87.0 °C whose atoms can occupy only two energy levels separated by 5.00x10 eV, where 1 eV (electron volt) is an energy unit equal to 1.60x1019 J. kg = 1.38x10-23 J/K , NA 6.02x1023 mol %3D Eg – a) Find the probability of occupation of the ground state with energy E1 and the 1st excited state with energy E2? b) How many atoms in the substance (on average) are in 5.00x10? eV the ground state and in the 1st excited state?
Consider 1.50 mols of a substance in thermal equilibrium at a temperature of 87.0 °C whose atoms can occupy only two energy levels separated by 5.00x10 eV, where 1 eV (electron volt) is an energy unit equal to 1.60x1019 J. kg = 1.38x10-23 J/K , NA 6.02x1023 mol %3D Eg – a) Find the probability of occupation of the ground state with energy E1 and the 1st excited state with energy E2? b) How many atoms in the substance (on average) are in 5.00x10? eV the ground state and in the 1st excited state?
Modern Physics
3rd Edition
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Chapter10: Statistical Physics
Section: Chapter Questions
Problem 6P
Related questions
Question
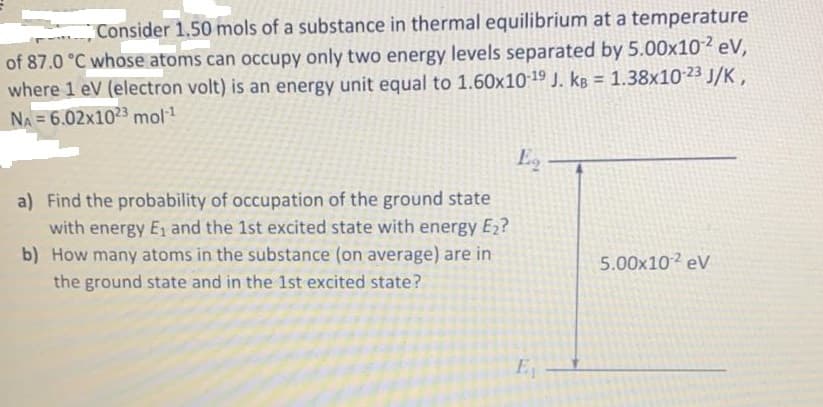
Transcribed Image Text:Consider 1.50 mols of a substance in thermal equilibrium at a temperature
of 87.0 °C whose atoms can occupy only two energy levels separated by 5.00x102 eV,
where 1 eV (electron volt) is an energy unit equal to 1.60x10 19 J. kB = 1.38x10 23 J/K ,
%3D
NA 6.02x1023 mol1
Eg
a) Find the probability of occupation of the ground state
with energy E1 and the 1st excited state with energy E2?
b) How many atoms in the substance (on average) are in
5.00x10 eV
the ground state and in the 1st excited state?
E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax