Consider again the system in quizzes 1 and 2, namely a particle moving in one dimension described by the normalized wavefunction V(x) = 30 х (а — х) for 0 <х <а and v(x) = 0 for x < 0 and x > a . a - a Determine the expectation value (p²) for the particle.
Consider again the system in quizzes 1 and 2, namely a particle moving in one dimension described by the normalized wavefunction V(x) = 30 х (а — х) for 0 <х <а and v(x) = 0 for x < 0 and x > a . a - a Determine the expectation value (p²) for the particle.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter11: Quantum Mechanics: Model Systems And The Hydrogen Atom
Section: Chapter Questions
Problem 11.61E: What is the physical explanation of the difference between a particle having the 3-D rotational...
Related questions
Question

Transcribed Image Text:Consider again the system in quizzes 1 and 2, namely a particle moving in one dimension
described by the normalized wavefunction
(x) =
30
1 (а — х) for 0 < х <a
and (x) = 0 for x < 0 and x > a .
а
Determine the expectation value () for the particle.
Expert Solution
Step 1
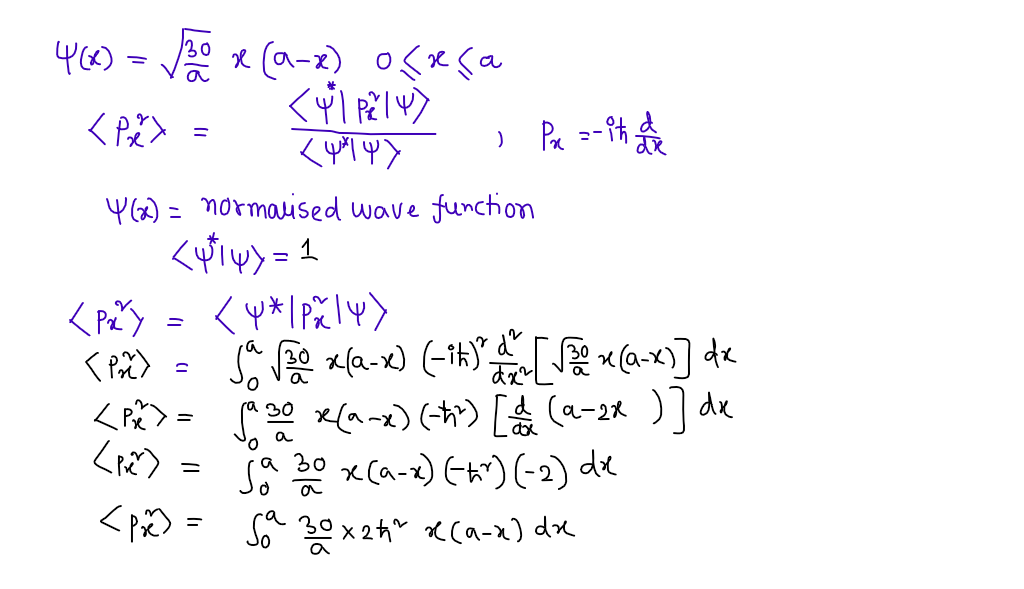
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning