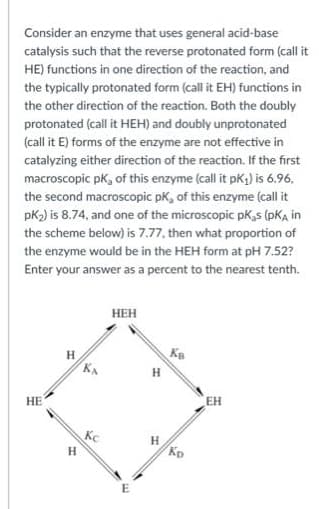
Consider an enzyme that uses general acid-base catalysis such that the reverse protonated form (call it HE) functions in one direction of the reaction, and the typically protonated form (call it EH) functions in the other direction of the reaction. Both the doubly protonated (call it HEH) and doubly unprotonated (call it E) forms of the enzyme are not effective in catalyzing either direction of the reaction. If the first macroscopic pk, of this enzyme (call it pK,) is 6.96. the second macroscopic pK, of this enzyme (call it pK2) is 8.74, and one of the microscopic pK,s (pKA in the scheme below) is 7.77, then what proportion of the enzyme would be in the HEH form at pH 7.52? Enter your answer as a percent to the nearest tenth.
Proteins
We generally tend to think of proteins only from a dietary lens, as a component of what we eat. However, they are among the most important and abundant organic macromolecules in the human body, with diverse structures and functions. Every cell contains thousands and thousands of proteins, each with specific functions. Some help in the formation of cellular membrane or walls, some help the cell to move, others act as messages or signals and flow seamlessly from one cell to another, carrying information.
Protein Expression
The method by which living organisms synthesize proteins and further modify and regulate them is called protein expression. Protein expression plays a significant role in several types of research and is highly utilized in molecular biology, biochemistry, and protein research laboratories.
correct answer should be 20.6 but how?
Trending now
This is a popular solution!
Step by step
Solved in 2 steps









