Consider Mg²+ and 02. Which do you predict is larger and why? 1. Mg²+ is larger because magnesium is farther to the left and farther down the periodic table than oxygen. 2. Mg²+ is larger because it contains more electrons so there are more electron-electron repulsions 3. Mg²+ and O²- are the same size because they both contain the same number of electrons so there is the same amount of electron-electron repulsion. 4. 0²- is larger because anions are always larger than cations. 5.0²- is larger because less protons are attracting the same number of electrons as Mg²+. ооооо
Consider Mg²+ and 02. Which do you predict is larger and why? 1. Mg²+ is larger because magnesium is farther to the left and farther down the periodic table than oxygen. 2. Mg²+ is larger because it contains more electrons so there are more electron-electron repulsions 3. Mg²+ and O²- are the same size because they both contain the same number of electrons so there is the same amount of electron-electron repulsion. 4. 0²- is larger because anions are always larger than cations. 5.0²- is larger because less protons are attracting the same number of electrons as Mg²+. ооооо
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 116QRT
Related questions
Question
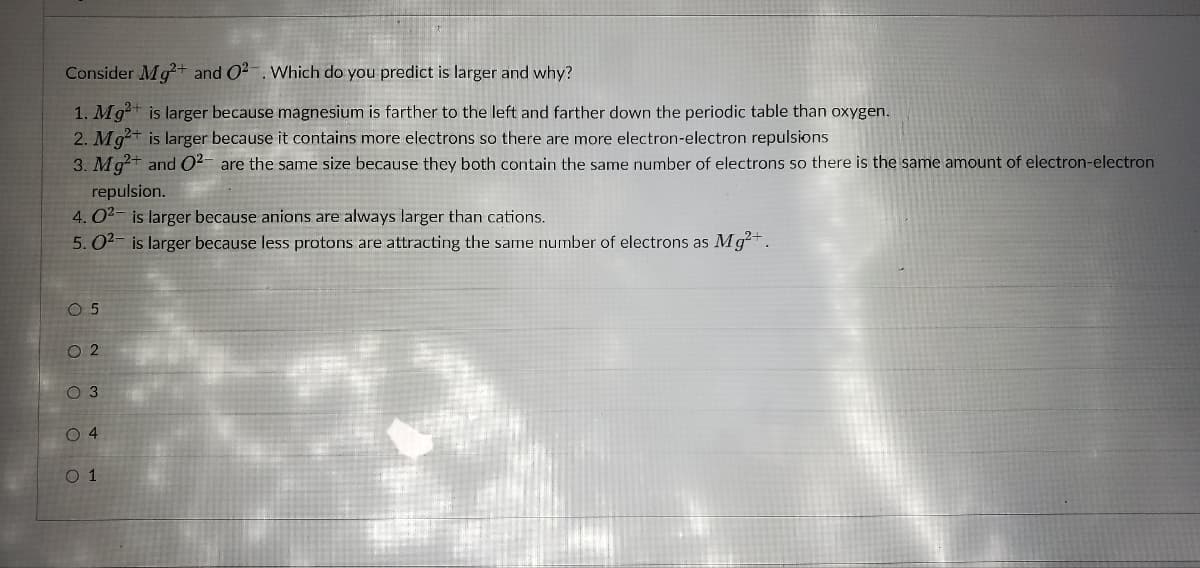
Transcribed Image Text:Consider Mg²+ and 02. Which do you predict is larger and why?
1. Mg²+ is larger because magnesium is farther to the left and farther down the periodic table than oxygen.
2. Mg²+ is larger because it contains more electrons so there are more electron-electron repulsions
3. Mg²+ and O²- are the same size because they both contain the same number of electrons so there is the same amount of electron-electron
repulsion.
4.0²- is larger because anions are always larger than cations.
5.0²- is larger because less protons are attracting the same number of electrons as Mg²+.
ο ο ο ο ο
0 5
w N
0 2
O 3
04
0 1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning