Consider the equilibrium system described by the chemical reaction below, which has a value of Kc equal to 4.10 × 10¬ª at a high temperature. If 0.20 mol N2 and 0.15 mol O2 react in a 1.0 L vessel, what will the equilibrium concentration of O2 be?
Consider the equilibrium system described by the chemical reaction below, which has a value of Kc equal to 4.10 × 10¬ª at a high temperature. If 0.20 mol N2 and 0.15 mol O2 react in a 1.0 L vessel, what will the equilibrium concentration of O2 be?
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter13: Fundamental Equilibrium Concepts
Section: Chapter Questions
Problem 12E: Show that the complete chemical equation, the total ionic equation, and the net ionic equation for...
Related questions
Question
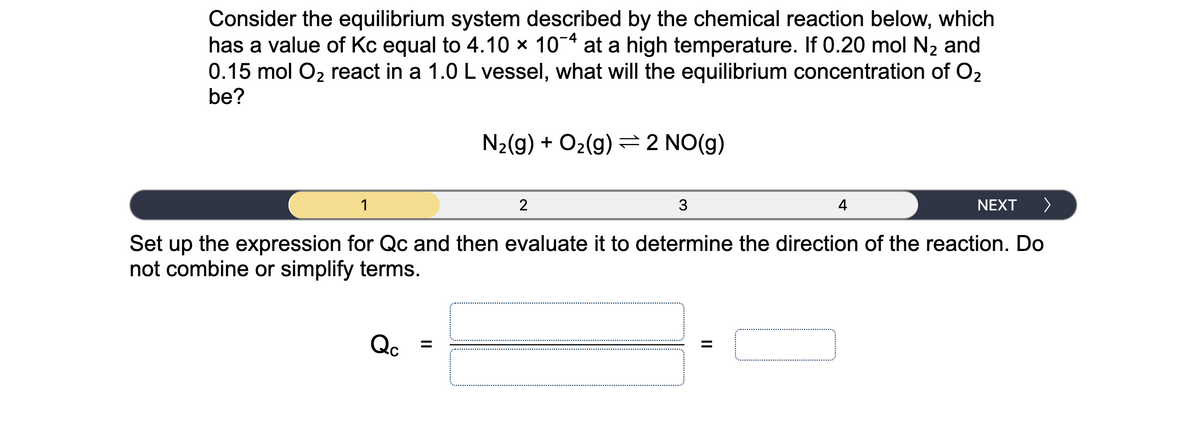
Transcribed Image Text:Consider the equilibrium system described by the chemical reaction below, which
has a value of Kc equal to 4.10 x 10-4 at a high temperature. If 0.20 mol N2 and
0.15 mol O2 react in a 1.0 L vessel, what will the equilibrium concentration of O2
be?
N2(g) + O2(g) =2 NO(g)
1
2
3
4
NEXT
>
Set up the expression for Qc and then evaluate it to determine the direction of the reaction. Do
not combine or simplify terms.
Qc
%3D
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning