Consider the following reaction: 2S03(g) 2S02(g) + O2(g) If 8.87x10-2 moles of SO (g), 0.541 moles of S02, and 0.279 moles of O, are at equilibrium in a 12.1 L container at 1.39x10 K, the value of the equilibrium constant, Ke, is
Consider the following reaction: 2S03(g) 2S02(g) + O2(g) If 8.87x10-2 moles of SO (g), 0.541 moles of S02, and 0.279 moles of O, are at equilibrium in a 12.1 L container at 1.39x10 K, the value of the equilibrium constant, Ke, is
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.15QAP
Related questions
Question
Transcribed Image Text:NLV2 | Online teachin
b Search results for 'A sti
Biology 2e
i (7) Biology of Organism
F Lauren Frias Review Qu
G Why are plasma membr
/takeAssignment/takeCovalentActivity.do
TripAdvisor f Facebook
Use the References to access important values if needed for this question.
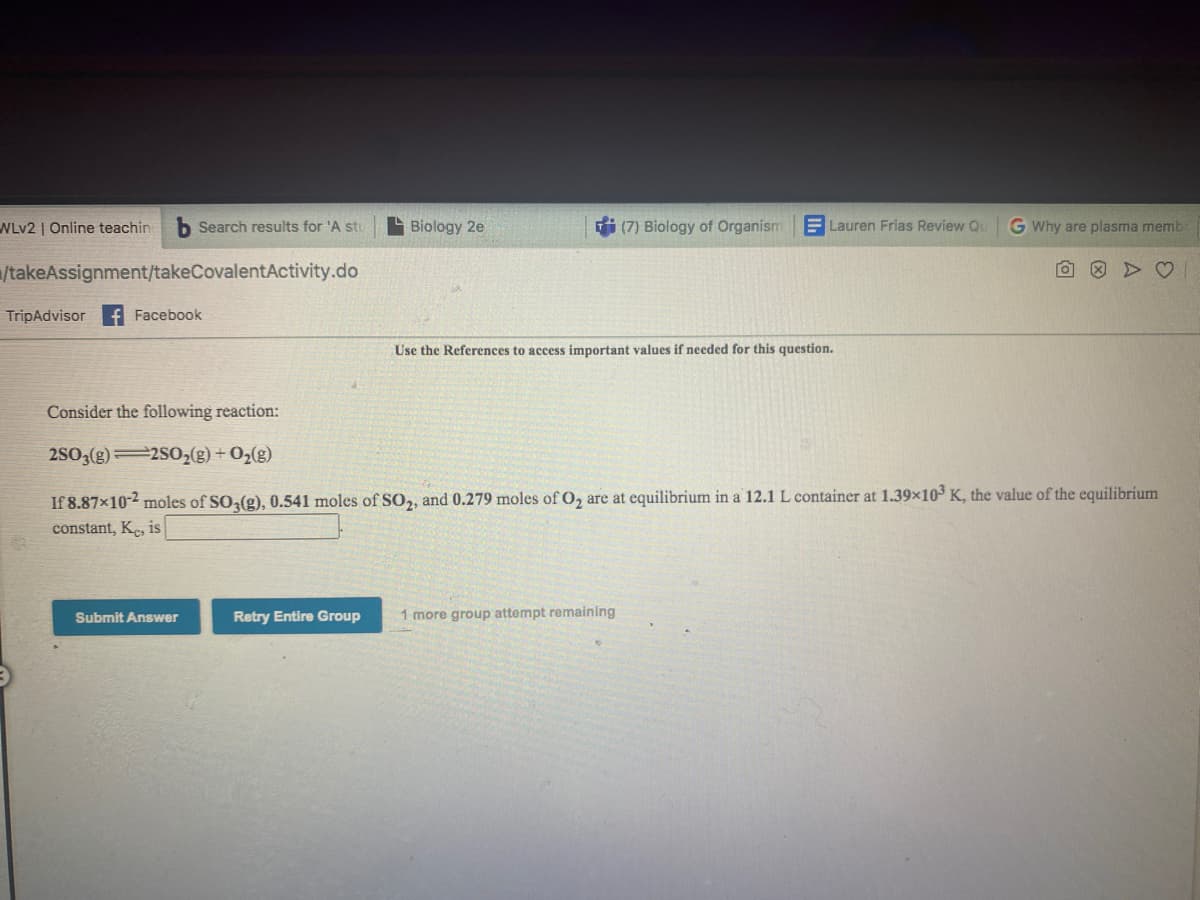
Consider the following reaction:
2S03(g)2S02(g) + O2(g)
If 8.87x10-2 moles of SO (g), 0.541 moles of S0,, and 0.279 moles of O, are at equilibrium in a 12.1 L container at 1.39x10 K, the value of the equilibrium
constant, Ke, is
Retry Entire Group
1 more group attempt remaining
Submit Answer
Expert Solution
Step 1
.jpg)
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax