Consider the following balanced equation: 2 H2 + 02 -2 H20 What mass of water (H20) will be collected if 20.0 grams of oxygen gas (02) are consumed?
Consider the following balanced equation: 2 H2 + 02 -2 H20 What mass of water (H20) will be collected if 20.0 grams of oxygen gas (02) are consumed?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 7RQ: Consider the hypothetical reaction between A2 and AB pictured below. What is the balanced equation?...
Related questions
Question
Consider the following balanced equation: 2 H2 + O2 → 2 H2O
What mass of water (H2O) will be collected if 20.0 grams of oxygen gas (O2) are consumed?
(Mass of H2O = 18.02 g/mol and Mass of O2 = 32.00 g/mol)
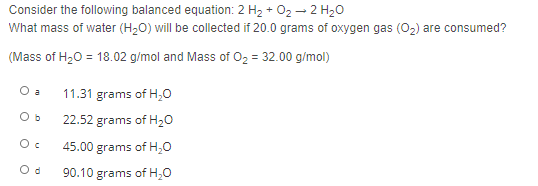
Transcribed Image Text:Consider the following balanced equation: 2 H2 + 02 – 2 H20
What mass of water (H,0) will be collected if 20.0 grams of oxygen gas (0,) are consumed?
(Mass of H20 = 18.02 g/mol and Mass of O2 = 32.00 g/mol)
O a
11.31 grams of H,0
22.52 grams of H20
45.00 grams of H,0
90.10 grams of H,0
Expert Solution
Step 1
From the given data,it is clear that
2 mol H2 +1 mol O2 → 2mol H2O
we know that ,1 mol of a compound = molar mass of that compound
molar mass of O2 = 32 g/mol
Then, 1 mol of O2 contains 32 g of O2
Also,molar mass of H2O= 18.02 g/mol
Then, 1 mol of H2O contains 18.02 g of H2O
:. 2 mol of H2O contains 2 X 18.02 g of H2O
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning