Consider the following data on some weak acids and weak bases: acid base K. к, olo name formula name formula acetic acid HCH;CO2 1.8 × 10 -5 -4 methylamine CH3NH2 4.4 x 10° Ar HNO2 4.5 x 10 ethylamine C2H;NH2 6.4 × 10 nitrous acid Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution pH 0.1 M NaCi 0.1 M NANO2 4 (highest) + 0.1 M KCH3CO2 3 0.1 M C2H5NH3B 1 (lowest) +
Consider the following data on some weak acids and weak bases: acid base K. к, olo name formula name formula acetic acid HCH;CO2 1.8 × 10 -5 -4 methylamine CH3NH2 4.4 x 10° Ar HNO2 4.5 x 10 ethylamine C2H;NH2 6.4 × 10 nitrous acid Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution pH 0.1 M NaCi 0.1 M NANO2 4 (highest) + 0.1 M KCH3CO2 3 0.1 M C2H5NH3B 1 (lowest) +
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 96QRT
Related questions
Question
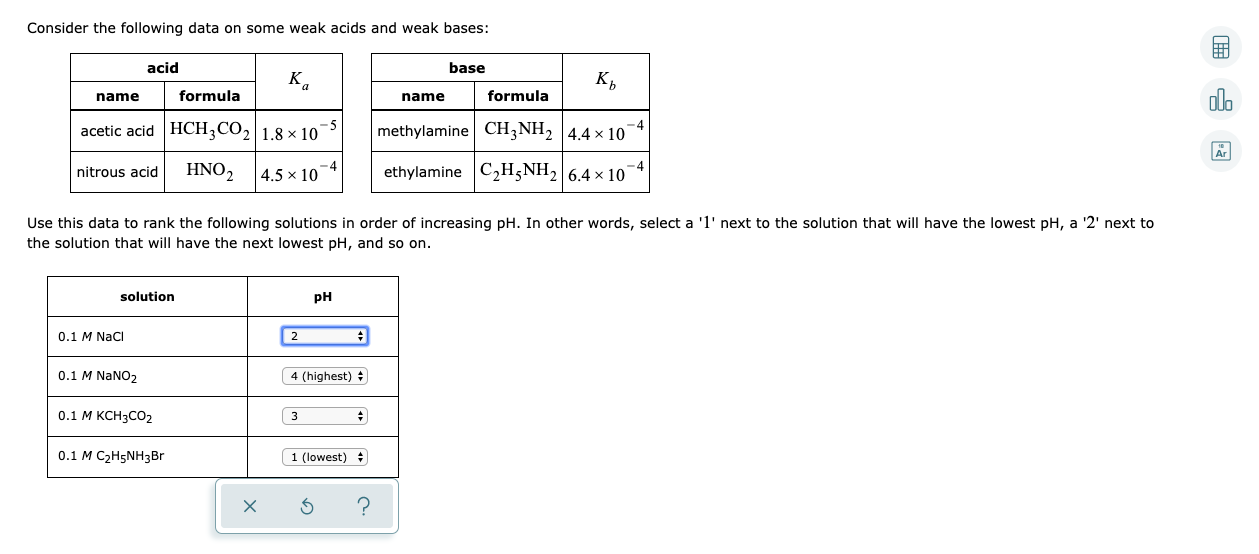
Transcribed Image Text:Consider the following data on some weak acids and weak bases:
acid
base
K.
к,
olo
name
formula
name
formula
acetic acid HCH;CO2 1.8 × 10
-5
-4
methylamine CH3NH2 4.4 x 10°
Ar
HNO2
4.5 x 10
ethylamine C2H;NH2 6.4 × 10
nitrous acid
Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to
the solution that will have the next lowest pH, and so on.
solution
pH
0.1 M NaCi
0.1 M NANO2
4 (highest) +
0.1 M KCH3CO2
3
0.1 M C2H5NH3B
1 (lowest) +
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning