Consider the following equilibrium process between dinitrogen tetrafluoride and nitrogen difluoride. N2F4 (g) 2 NF2 (g) AH° = - 38.5 kJ/mol By expanding the expression for Kp for this reaction explain precisely what happens to the position of equilibrium when (i) the total pressure is increased, (ii) partial pressure of N2F4 is decreased.
Consider the following equilibrium process between dinitrogen tetrafluoride and nitrogen difluoride. N2F4 (g) 2 NF2 (g) AH° = - 38.5 kJ/mol By expanding the expression for Kp for this reaction explain precisely what happens to the position of equilibrium when (i) the total pressure is increased, (ii) partial pressure of N2F4 is decreased.
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.27PAE
Related questions
Question
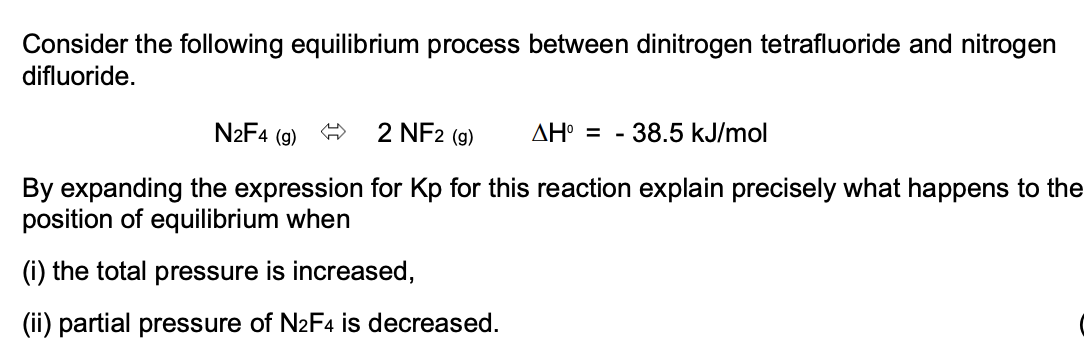
Transcribed Image Text:Consider the following equilibrium process between dinitrogen tetrafluoride and nitrogen
difluoride.
N2F4 (g)
2 NF2 (g)
AH° = - 38.5 kJ/mol
By expanding the expression for Kp for this reaction explain precisely what happens to the
position of equilibrium when
(i) the total pressure is increased,
(ii) partial pressure of N2F4 is decreased.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning