Consider the following half-reactions: I2(s) + 2e- → 2 F(aq), E°(red) = 0.54 V, Cd²"(aq) + 2 e- → Cd(s), E°(red) = -0.40 V. Will the electrochemical cell represented by IF | l2 || Cd²" | Cd be galvanic or electrolytic under standard conditions? A) Galvanic B) Electrolytic C) Not enough information
Consider the following half-reactions: I2(s) + 2e- → 2 F(aq), E°(red) = 0.54 V, Cd²"(aq) + 2 e- → Cd(s), E°(red) = -0.40 V. Will the electrochemical cell represented by IF | l2 || Cd²" | Cd be galvanic or electrolytic under standard conditions? A) Galvanic B) Electrolytic C) Not enough information
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter24: Coulometry
Section: Chapter Questions
Problem 24.4QAP: Halide ions can he deposited at a silver anode, the reaction being Ag(s) + X- AgX(s) +e- Suppose...
Related questions
Question
Transcribed Image Text:Evolution
Chemisty
를 Op
늘 17.
늘 Ch.
들 An:
S Sol
101
b Sea
Co
Lab
Email
Searches
Co
Sol
Titi
i app.101edu.co
Apps
School
SWGOH
MSF
SWTOR
Amazon
Noodle
Bills
Possible Purchases
Reading list
Question 10 of 15
Submit
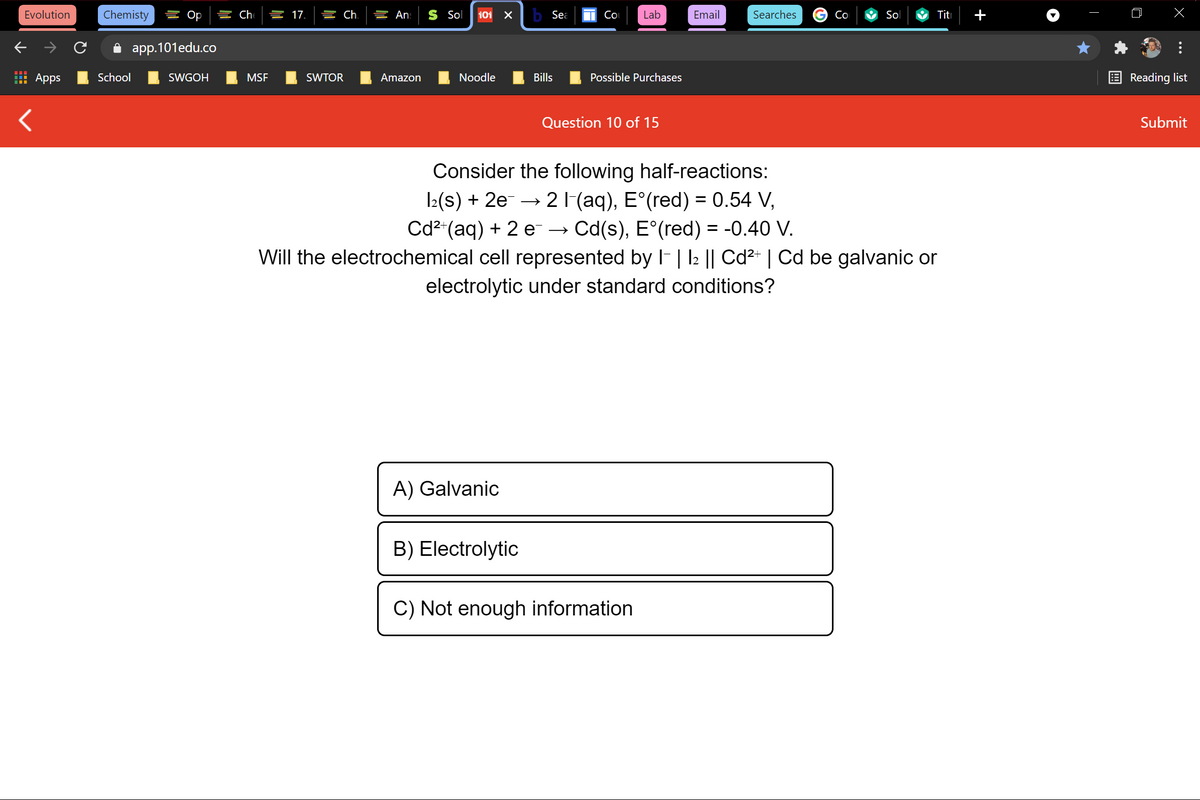
Consider the following half-reactions:
1:(s) + 2e- →
2 1(aq), E°(red) = 0.54 V,
Cd2"(aq) + 2 e → Cd(s), E°(red) = -0.40 V.
Will the electrochemical cell represented by F| l2 || Cd2- | Cd be galvanic or
electrolytic under standard conditions?
A) Galvanic
B) Electrolytic
C) Not enough information
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

