Consider the following hypothetical molecular collisions and predict which of the following will form potential products, given the values for the energy of activation, Ea, and enthalpy of the reaction, AH, in combination with the molecular orientation. Check all that apply. • View Available Hint(s) E,=7.55 kJ, AH = +9.56 kJ E, = 6.36 kJ, AH=-3.58 kJ ΕE-0, ΔΗ=+1.28 Cl o Cl o NO E,= 4.58 kJ, AH = +5.38 kJ E, = 2.48 kJ, AH =-5.98 kJ FF
Consider the following hypothetical molecular collisions and predict which of the following will form potential products, given the values for the energy of activation, Ea, and enthalpy of the reaction, AH, in combination with the molecular orientation. Check all that apply. • View Available Hint(s) E,=7.55 kJ, AH = +9.56 kJ E, = 6.36 kJ, AH=-3.58 kJ ΕE-0, ΔΗ=+1.28 Cl o Cl o NO E,= 4.58 kJ, AH = +5.38 kJ E, = 2.48 kJ, AH =-5.98 kJ FF
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter11: Rate Of Reaction
Section: Chapter Questions
Problem 99QAP: The gas-phase reaction between hydrogen and iodine H2(g)+I22HI(g)proceeds with a rate constant for...
Related questions
Question
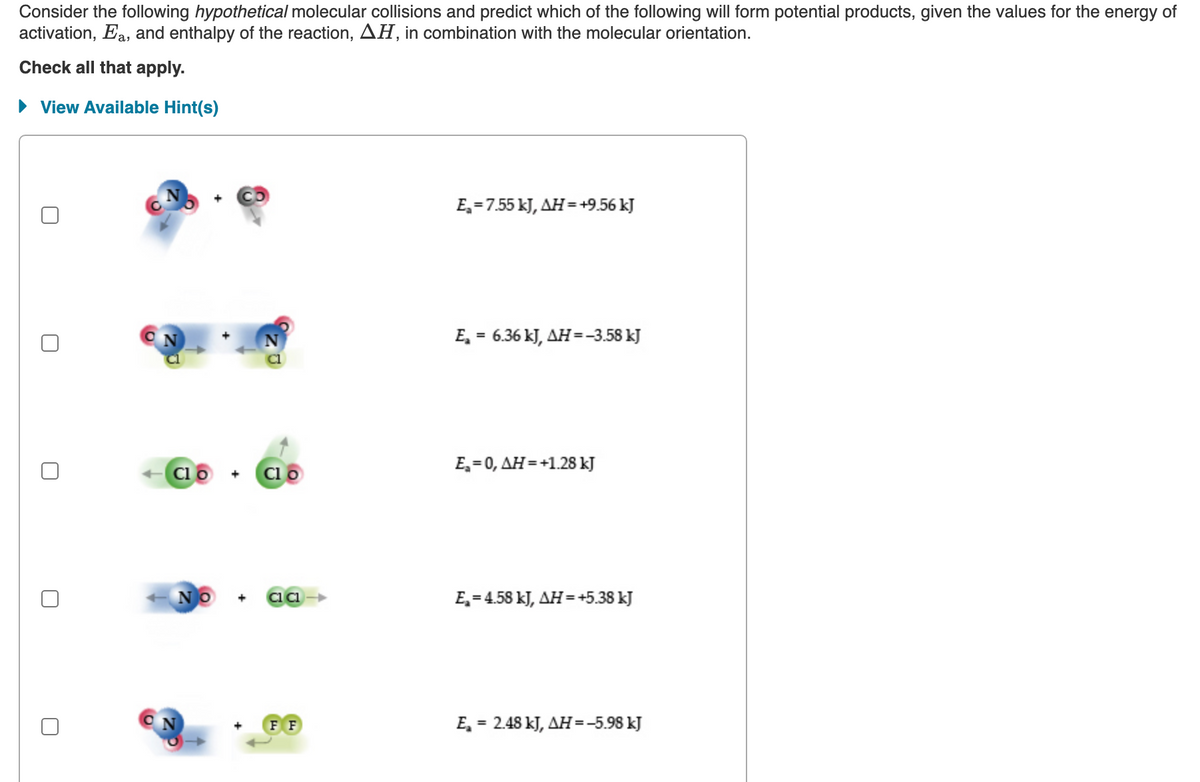
Transcribed Image Text:Consider the following hypothetical molecular collisions and predict which of the following will form potential products, given the values for the energy of
activation, Ea, and enthalpy of the reaction, AH, in combination with the molecular orientation.
Check all that apply.
• View Available Hint(s)
E,=7.55 kJ, AH = +9.56 kJ
E, = 6.36 kJ, AH=-3.58 kJ
%3D
Clo +
Clo
E,= 0, AH=+1.28 kJ
NO
E, = 4.58 kJ, AH =+5.38 kJ
F F
E, = 2.48 kJ, AH=-5.98 kJ
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,