Consider the following peptide. Gly-Lys-Ala-Val-Asp-Gly-lle-Val-Lys-Ala-Gly-His-Glu-Ala a) What would be net charge (positive or negative) on the peptide at pH 7.0? b) The peptide known to have some therapeutic value only if it has no overall charge at pH 7.0. Assuming that the single amino acid replacement does not alter the therapeutic potential of the peptide, which amino acid replacement do you suggest so peptide has no charge at pH 7.0? Explain your answer. Write three most important characteristics of a peptide bond.
Consider the following peptide. Gly-Lys-Ala-Val-Asp-Gly-lle-Val-Lys-Ala-Gly-His-Glu-Ala a) What would be net charge (positive or negative) on the peptide at pH 7.0? b) The peptide known to have some therapeutic value only if it has no overall charge at pH 7.0. Assuming that the single amino acid replacement does not alter the therapeutic potential of the peptide, which amino acid replacement do you suggest so peptide has no charge at pH 7.0? Explain your answer. Write three most important characteristics of a peptide bond.
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
i need the answer quickly
Transcribed Image Text:1.
Consider the following peptide.
Gly-Lys-Ala-Val-Asp-Gly-lle-Val-Lys-Ala-Gly-His-Glu-Ala
a) What would be net charge (positive or negative) on the peptide at pH 7.0?
b) The peptide known to have some therapeutic value only if it has no overall charge at pH 7.0.
Assuming that the single amino acid replacement does not alter the therapeutic potential of the
peptide, which amino acid replacement do you suggest so peptide has no charge at pH 7.0? Explain
your answer.
2
Write three most important characteristics of a peptide bond.
3
Explain change in Entropy and Enthalpy during process of protein folding.
4
Write short note on the following.
(a) Ramachandran Plot
(c) Zwitterion of amino acid
(b) a-helix of protein
(d) Levinthal Paradox of protein folding
List four major differences of protein folding invitro versus folding in the cell.
What are molecular Chaperones? What do you mean by Kinetic partitioning? Explain the
conditions under which chaperones act as Holdase, foldase, and Unfoldase?
What are the characteristics of amyloid fibrils (formed as a result of Inter-molecular Interaction).
Do you think such a structural feature can be capitalized for development of drugs that can be
used to treat two diseases involving aggregation of two different proteins?
8
6
It is known that native proteins are only marginally stable (-0.4KJ/amino acid). Explain its
significance
How does glycosylation affect the physico-chemical properties of proteins?
Suppose that a protein contains six potential N-linked glycosylation sites. How
many possible proteins can be generated, depending on which of these sites is actually
glycosylated? Do not include the effects of diversity within the carbohydrate added.
10
11
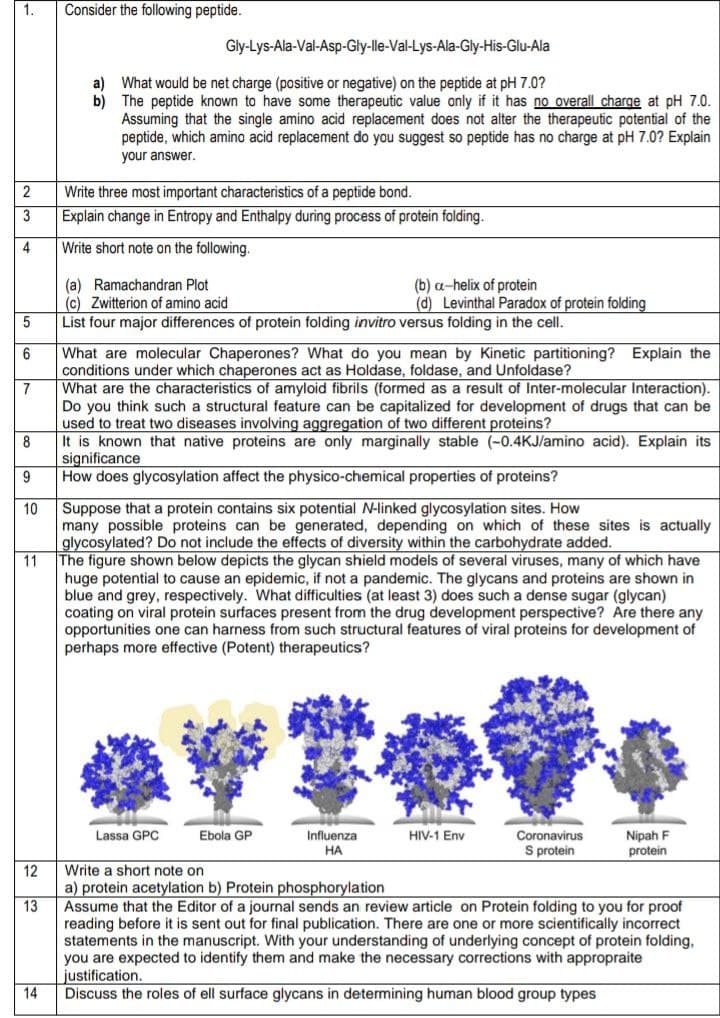
The figure shown below depicts the glycan shield models of several viruses, many of which have
huge potential to cause an epidemic, if not a pandemic. The glycans and proteins are shown in
blue and grey, respectively. What difficulties (at least 3) does such a dense sugar (glycan)
coating on viral protein surfaces present from the drug development perspective? Are there any
opportunities one can harness from such structural features of viral proteins for development of
perhaps more effective (Potent) therapeutics?
Lassa GPC
Nipah F
protein
Ebola GP
Influenza
НА
HIV-1 Env
Coronavirus
S protein
Write a short note on
12
a) protein acetylation b) Protein phosphorylation
13
Assume that the Editor of a journal sends an review article on Protein folding to you for proof
reading before it is sent out for final publication. There are one or more scientifically incorrect
statements in the manuscript. With your understanding of underlying concept of protein folding,
you are expected to identify them and make the necessary corrections with appropraite
justification.
Discuss the roles of ell surface glycans in determining human blood group types
14
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON