Consider the following statements about first ionization energies: I. Because the effective nuclear charge for Mg is greater than that for Be, the first ionization energy of Mg is greater than that of Be. II. The first ionization energy of O is less than that of N because in O we must pair electrons in the 2p orbitals. III. The first ionization energy of Ar is less than that of Ne because a 3p electron in Ar is farther from the nucleus than a 2p electron in Ne. Which of the statements I, II, and III is or are true? O Only one of the statements is true O Statements I and Il are true O Statements I and II are true Statements Il and IIlI are true O All three statements are true
Consider the following statements about first ionization energies: I. Because the effective nuclear charge for Mg is greater than that for Be, the first ionization energy of Mg is greater than that of Be. II. The first ionization energy of O is less than that of N because in O we must pair electrons in the 2p orbitals. III. The first ionization energy of Ar is less than that of Ne because a 3p electron in Ar is farther from the nucleus than a 2p electron in Ne. Which of the statements I, II, and III is or are true? O Only one of the statements is true O Statements I and Il are true O Statements I and II are true Statements Il and IIlI are true O All three statements are true
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section: Chapter Questions
Problem 11ALQ: r Questions 11—13, you will need to consider ionizations beyond the first ionization energy. For...
Related questions
Question
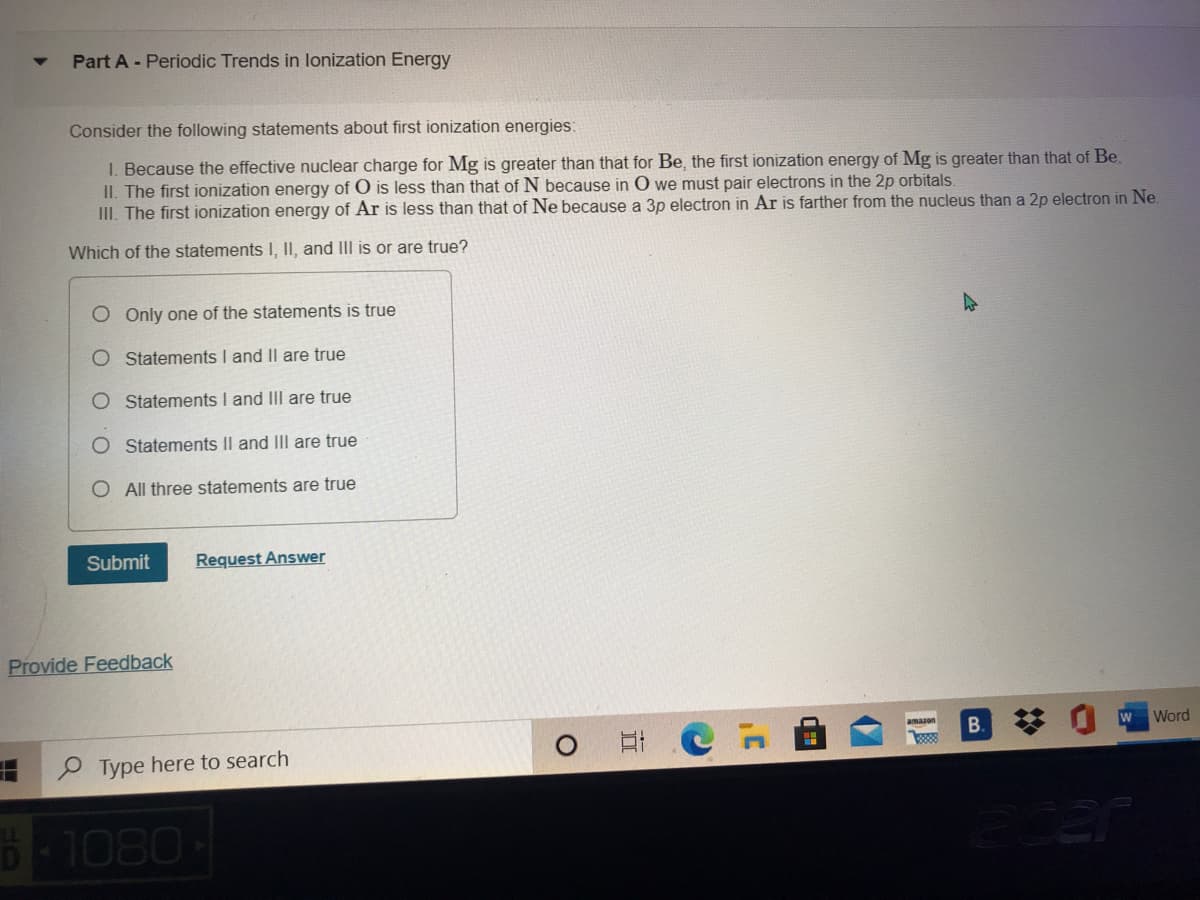
Transcribed Image Text:Part A - Periodic Trends in lonization Energy
Consider the following statements about first ionization energies:
I. Because the effective nuclear charge for Mg is greater than that for Be, the first ionization energy of Mg is greater than that of Be.
II. The first ionization energy of O is less than that of N because in O we must pair electrons in the 2p orbitals.
III. The first ionization energy of Ar is less than that of Ne because a 3p electron in Ar is farther from the nucleus than a 2p electron in Ne.
Which of the statements , II, and III is or are true?
O Only one of the statements is true
O Statements I and Il are true
Statements I and III are true
Statements Il and III are true
O All three statements are true
Submit
Request Answer
Provide Feedback
O #Cn I
B.
Word
amazon
3000
P Type here to search
B-1080
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning