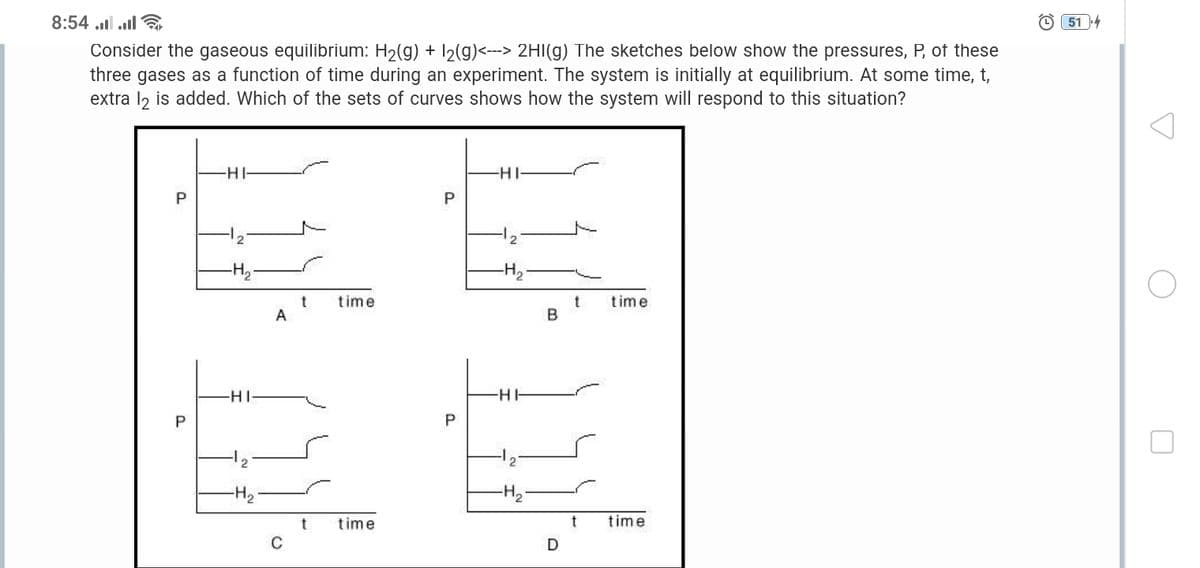
Consider the gaseous equilibrium: H2(g) + 12(g)<--> 2HI(g) The sketches below show the pressures, P, of these three gases as a function of time during an experiment. The system is initially at equilibrium. At some time, t, extra l2 is added. Which of the sets of curves shows how the system will respond to this situation? -HI- -HI- P 12 -H2- -H2 time time A B -HI- -HH -H2 -H2 time time C D P. P. P.
Consider the gaseous equilibrium: H2(g) + 12(g)<--> 2HI(g) The sketches below show the pressures, P, of these three gases as a function of time during an experiment. The system is initially at equilibrium. At some time, t, extra l2 is added. Which of the sets of curves shows how the system will respond to this situation? -HI- -HI- P 12 -H2- -H2 time time A B -HI- -HH -H2 -H2 time time C D P. P. P.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter13: Chemical Equilibrium
Section: Chapter Questions
Problem 11ALQ: Explain why the development of a vapor pressure above a liquid in a closed container represents an...
Related questions
Question
Transcribed Image Text:8:54 .l .l
51 ):4
Consider the gaseous equilibrium: H2(g) + I2(g)<---> 2HI(g) The sketches below show the pressures, P, of these
three gases as a function of time during an experiment. The system is initially at equilibrium. At some time, t,
extra l2 is added. Which of the sets of curves shows how the system will respond to this situation?
-H-
-HI
P
P
time
time
A
-H-
-H-
t
time
time
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning