Mercury and oxygen react to form mercury(II) oxide, like this: 2 Hg(1)+O2(g)→2 HgO(s) At a certain temperature, a chemist finds that a 7.0 L reaction vessel containing a mixture of mercury, oxygen, and mercury(II) oxide at equilibrium has the following composition: compound amount Hg 7.0 g O2 5.8 g HgO 16.5 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits.
Mercury and oxygen react to form mercury(II) oxide, like this: 2 Hg(1)+O2(g)→2 HgO(s) At a certain temperature, a chemist finds that a 7.0 L reaction vessel containing a mixture of mercury, oxygen, and mercury(II) oxide at equilibrium has the following composition: compound amount Hg 7.0 g O2 5.8 g HgO 16.5 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter15: Principles Of Chemical Reactivity: Equilibria
Section: Chapter Questions
Problem 7PS: The reaction PCl5(g) PCl3(g) + Cl2(g) was examined at 250 C. At equilibrium, [PCl5] = 4.2 105...
Related questions
Question
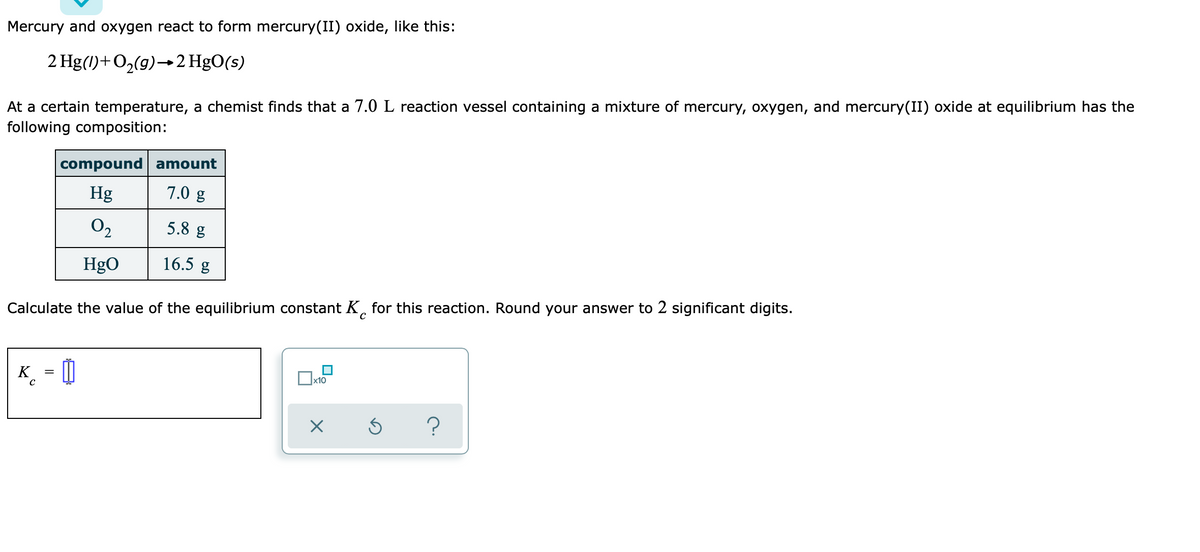
Transcribed Image Text:Mercury and oxygen react to form mercury(II) oxide, like this:
2 Hg(1)+O2(g)→2 HgO(s)
At a certain temperature, a chemist finds that a 7.0 L reaction vessel containing a mixture of mercury, oxygen, and mercury(II) oxide at equilibrium has the
following composition:
compound amount
Hg
7.0 g
O2
5.8 g
HgO
16.5 g
Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits.
K_ = |
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning