Consider the hypothetical reaction A(g) + B(g) ----> C(g) The four containers below represent this reaction being run with different initial amounts of A and B. Assume that the volume of each container is 1.0L. The reaction is second order with respect to A and first order in respect to B. Which of the containers would have the lowest reaction rate? Explain Which would have the highest reaction rate?
Consider the hypothetical reaction A(g) + B(g) ----> C(g) The four containers below represent this reaction being run with different initial amounts of A and B. Assume that the volume of each container is 1.0L. The reaction is second order with respect to A and first order in respect to B. Which of the containers would have the lowest reaction rate? Explain Which would have the highest reaction rate?
Chapter12: Chemical Kinetics
Section: Chapter Questions
Problem 44E: The rate of the reaction O(g)+NO2(g)NO(g)+O2(g) was studied at a certain temperature. a. In one...
Related questions
Question
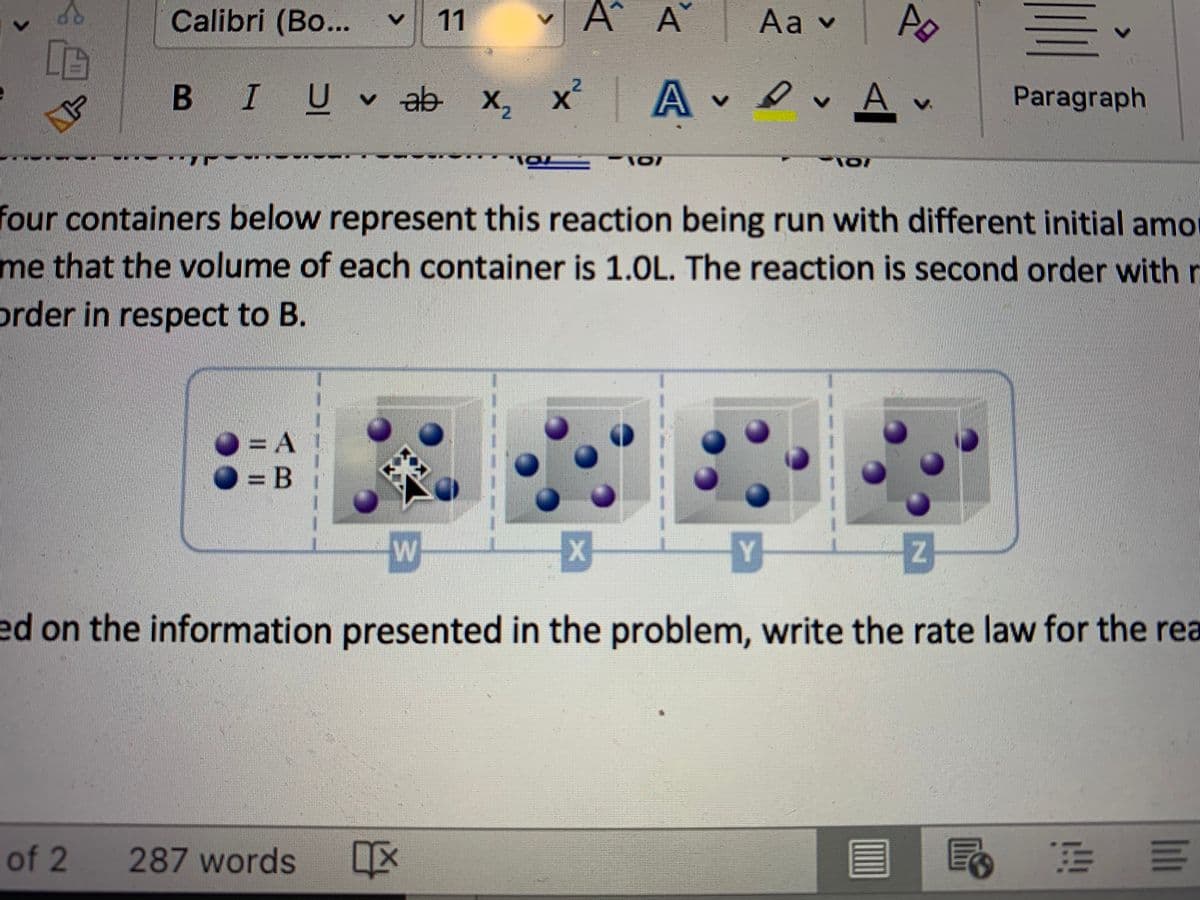
Consider the hypothetical reaction A(g) + B(g) ----> C(g)
The four containers below represent this reaction being run with different initial amounts of A and B. Assume that the volume of each container is 1.0L. The reaction is second order with respect to A and first order in respect to B.
Which of the containers would have the lowest reaction rate? Explain
Which would have the highest reaction rate?
Transcribed Image Text:Calibri (Bo...
11
A
Aav
B IU ab x,
x² A v
v evAv
X2
Paragraph
four containers below represent this reaction being run with different initial amo
me that the volume of each container is 1.0L. The reaction is second order with
prder in respect to B.
O =DA
O = B
Y
ed on the information presented in the problem, write the rate law for the rea
of 2 287 words
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning