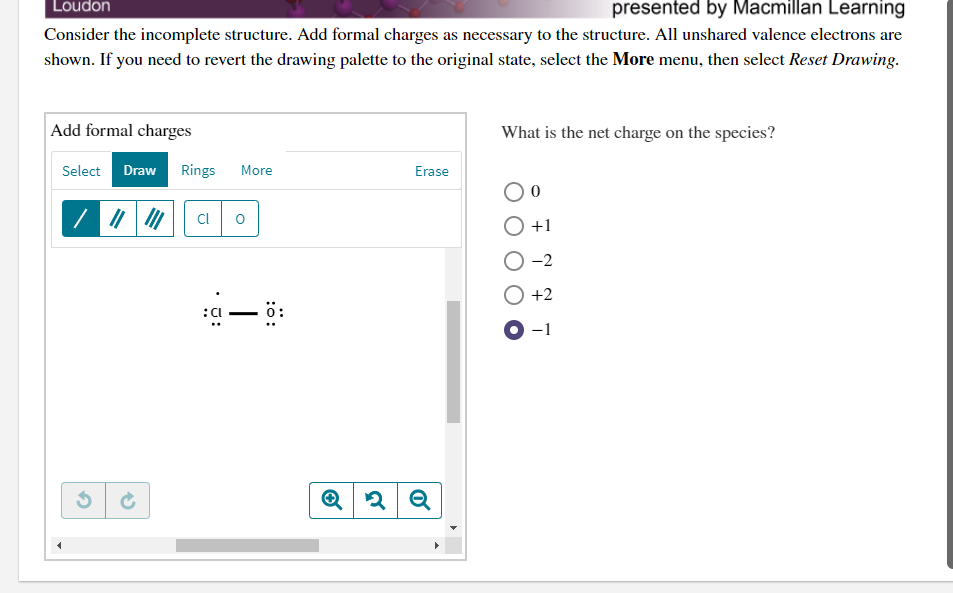
Consider the incomplete structure. Add formal charges as necessary to the structure. All unshared valence electrons are shown. If you need to revert the drawing palette to the original state, select the More menu, then select Reset Drawing. Add formal charges What is the net charge on the species? Select Draw Rings More Erase O+1 O-2 O +2 O-1 :ö:
Consider the incomplete structure. Add formal charges as necessary to the structure. All unshared valence electrons are shown. If you need to revert the drawing palette to the original state, select the More menu, then select Reset Drawing. Add formal charges What is the net charge on the species? Select Draw Rings More Erase O+1 O-2 O +2 O-1 :ö:
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 90AP: Two possible Lewis diagrams for sulfine (H2CSO) are (a) Compute the formal charges on all atoms. (b)...
Related questions
Question
How do I draw the completed structure?
Thanks
Transcribed Image Text:Loudon
presented by Macmillan Learning
Consider the incomplete structure. Add formal charges as necessary to the structure. All unshared valence electrons are
shown. If you need to revert the drawing palette to the original state, select the More menu, then select Reset Drawing.
Add formal charges
What is the net charge on the species?
Select Draw
Rings
More
Erase
Cl
O +1
-2
+2
-1
:ö:
Expert Solution
Step 1
Formal charge = no of valance electrons - (no of covalent bonds + no of lone pair electrons)
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning