H. A. Determine the formal charge of oxygen in the structure. If the atom is formally neutral, indicate a charge of zero. H. B. Draw an alternative Lewis structure or resonance structure for the incomplete structure. Show the unshared electron pairs and nonzero formal charges in your structure. Don't use radicals. Select Draw Rings More Erase H C
H. A. Determine the formal charge of oxygen in the structure. If the atom is formally neutral, indicate a charge of zero. H. B. Draw an alternative Lewis structure or resonance structure for the incomplete structure. Show the unshared electron pairs and nonzero formal charges in your structure. Don't use radicals. Select Draw Rings More Erase H C
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter2: Lewis Structures
Section: Chapter Questions
Problem 20CTQ
Related questions
Question
Answer part a and b
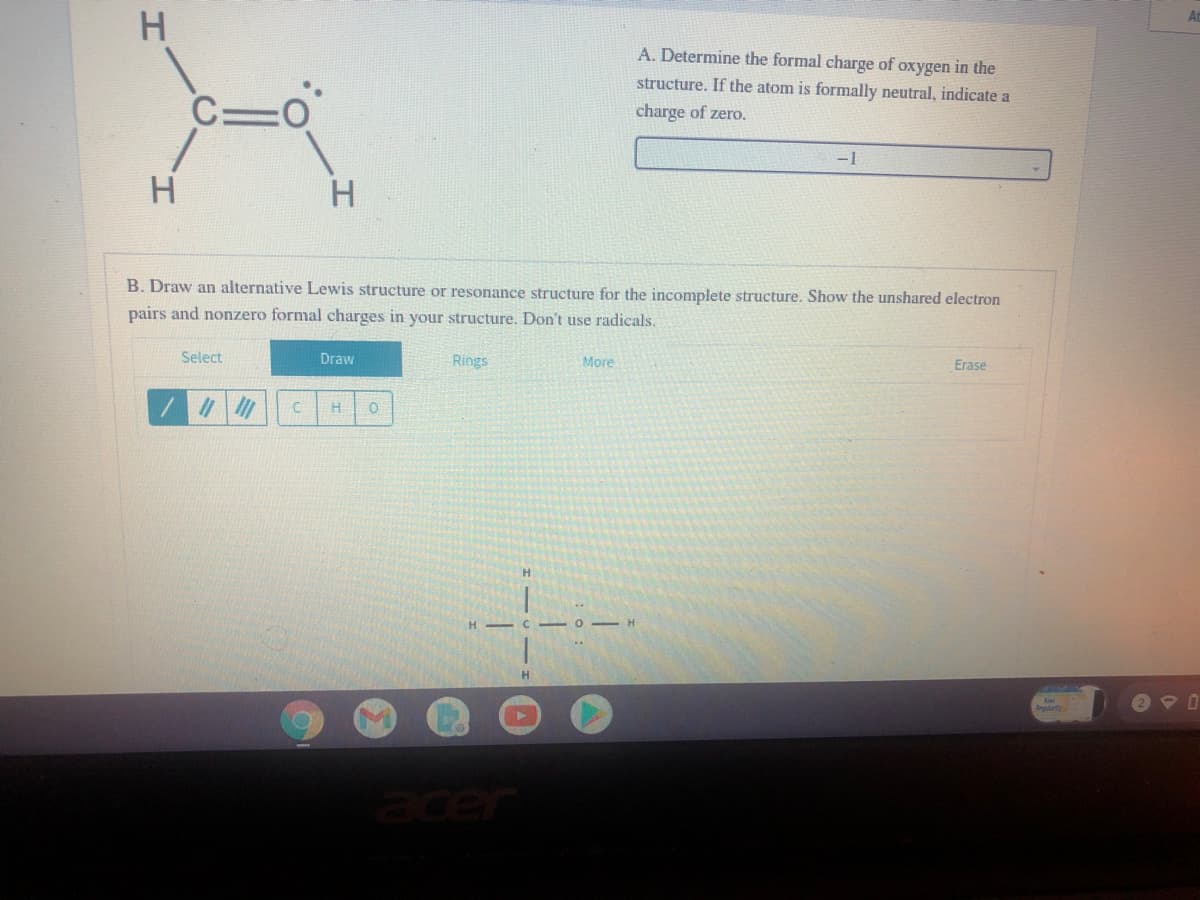
Transcribed Image Text:H.
A. Determine the formal charge of oxygen in the
structure. If the atom is formally neutral, indicate a
C=0
charge of zero.
-1
H.
B. Draw an alternative Lewis structure or resonance structure for the incomplete structure. Show the unshared electron
pairs and nonzero formal charges in your structure. Don't use radicals.
Select
Draw
Rings
More
Erase
Brguarty
acer
H.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning