Consider the insoluble compound aluminum phosphate , AlIPO4. The aluminum ion also forms a complex with hydroxide ions . Write a balanced net ionic equation to show why the solubility of AIPO, (s) increases in the presence of hydroxide ions and calculate the equilibrium constant for this reaction, For Al(OH)4 , K= 7.7x1033. Use the pull-down boxes to specify states such as (aq) or (s). K = Consider the insoluble compound copper(II) hydroxide , Cu(OH), . The copper(II) ion also forms a complex with ammonia . Write a balanced net ionic equation to show why the solubility of Cu(OH), (s) increases in the presence of ammonia and calculate the equilibrium constant for this reaction. For Cu(NH3),* , Kf= 6.8×1012 . Use the pull-down boxes to specify states such as (aq) or (s). 2 Cu(NH3), 2+ Cu(OH)2 + |4NH3 ( (aq) 3 / 2 Cu(NH,)"| (s) (aq) 20Н (aq) K= 1.36x10^-6 Incorrect Feedback: Partially Correct Incorrect Correct Answer(s): (s) in water and is the equation for the equilibrium in a saturated solution of Cu(OH)2 - The second step represems tne rormauon or me ammonia complex from the Cu2* ion in solution. As the Cu(NH3),* complex forms, Cu²* ion The first step below 1.1*10^-6 is removed from solution and the dissolution equilibrium shifts to the right. The sum of these two equations gives the desired equation, with an equilibrium constant given by the product of the K's for the first two. Cu(ОН)2 (8) 2 Cu²* (aq) + 2 OH" (aq) Кр — 1.6х10-19 Cu2* (aq) + 4 NH3 (aq) 2 Cu(NH3),* (aq) Kf= 6.8×1012 !! Cu(OH)2(s) + 4NH3(aq) 2 Cu(NH3),*(aq) + 20H°(aq) Knet = Kp X Kf=1.1×106 Note that Knet >> Ksp indicating a substantial increase in the solubility of Cu(OH), (s). (Previous Next
Consider the insoluble compound aluminum phosphate , AlIPO4. The aluminum ion also forms a complex with hydroxide ions . Write a balanced net ionic equation to show why the solubility of AIPO, (s) increases in the presence of hydroxide ions and calculate the equilibrium constant for this reaction, For Al(OH)4 , K= 7.7x1033. Use the pull-down boxes to specify states such as (aq) or (s). K = Consider the insoluble compound copper(II) hydroxide , Cu(OH), . The copper(II) ion also forms a complex with ammonia . Write a balanced net ionic equation to show why the solubility of Cu(OH), (s) increases in the presence of ammonia and calculate the equilibrium constant for this reaction. For Cu(NH3),* , Kf= 6.8×1012 . Use the pull-down boxes to specify states such as (aq) or (s). 2 Cu(NH3), 2+ Cu(OH)2 + |4NH3 ( (aq) 3 / 2 Cu(NH,)"| (s) (aq) 20Н (aq) K= 1.36x10^-6 Incorrect Feedback: Partially Correct Incorrect Correct Answer(s): (s) in water and is the equation for the equilibrium in a saturated solution of Cu(OH)2 - The second step represems tne rormauon or me ammonia complex from the Cu2* ion in solution. As the Cu(NH3),* complex forms, Cu²* ion The first step below 1.1*10^-6 is removed from solution and the dissolution equilibrium shifts to the right. The sum of these two equations gives the desired equation, with an equilibrium constant given by the product of the K's for the first two. Cu(ОН)2 (8) 2 Cu²* (aq) + 2 OH" (aq) Кр — 1.6х10-19 Cu2* (aq) + 4 NH3 (aq) 2 Cu(NH3),* (aq) Kf= 6.8×1012 !! Cu(OH)2(s) + 4NH3(aq) 2 Cu(NH3),*(aq) + 20H°(aq) Knet = Kp X Kf=1.1×106 Note that Knet >> Ksp indicating a substantial increase in the solubility of Cu(OH), (s). (Previous Next
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 70AP
Related questions
Question
Attached how to solve and questions
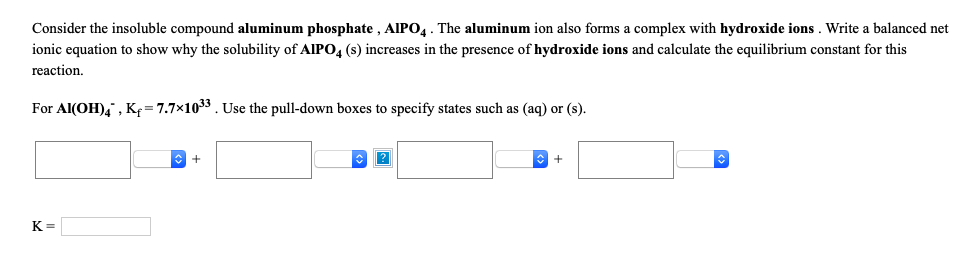
Transcribed Image Text:Consider the insoluble compound aluminum phosphate , AlIPO4. The aluminum ion also forms a complex with hydroxide ions . Write a balanced net
ionic equation to show why the solubility of AIPO, (s) increases in the presence of hydroxide ions and calculate the equilibrium constant for this
reaction,
For Al(OH)4 , K= 7.7x1033. Use the pull-down boxes to specify states such as (aq) or (s).
K =
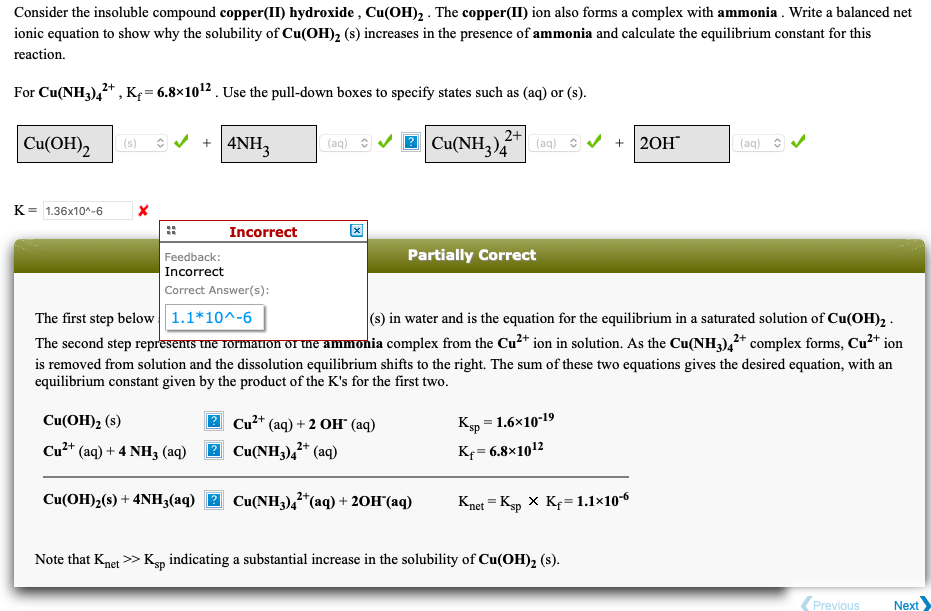
Transcribed Image Text:Consider the insoluble compound copper(II) hydroxide , Cu(OH), . The copper(II) ion also forms a complex with ammonia . Write a balanced net
ionic equation to show why the solubility of Cu(OH), (s) increases in the presence of ammonia and calculate the equilibrium constant for this
reaction.
For Cu(NH3),* , Kf= 6.8×1012 . Use the pull-down boxes to specify states such as (aq) or (s).
2 Cu(NH3),
2+
Cu(OH)2
+ |4NH3
( (aq) 3 / 2 Cu(NH,)"|
(s)
(aq)
20Н
(aq)
K= 1.36x10^-6
Incorrect
Feedback:
Partially Correct
Incorrect
Correct Answer(s):
(s) in water and is the equation for the equilibrium in a saturated solution of Cu(OH)2 -
The second step represems tne rormauon or me ammonia complex from the Cu2* ion in solution. As the Cu(NH3),* complex forms, Cu²* ion
The first step below 1.1*10^-6
is removed from solution and the dissolution equilibrium shifts to the right. The sum of these two equations gives the desired equation, with an
equilibrium constant given by the product of the K's for the first two.
Cu(ОН)2 (8)
2 Cu²* (aq) + 2 OH" (aq)
Кр — 1.6х10-19
Cu2* (aq) + 4 NH3 (aq)
2 Cu(NH3),* (aq)
Kf= 6.8×1012
!!
Cu(OH)2(s) + 4NH3(aq) 2 Cu(NH3),*(aq) + 20H°(aq)
Knet = Kp X Kf=1.1×106
Note that Knet >> Ksp indicating a substantial increase in the solubility of Cu(OH), (s).
(Previous
Next
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning