Consider the melting of ice at 0°C represented by the following equilibrium: H2Ols) H2O() The AS° for this process should be O negative zero O positive
Consider the melting of ice at 0°C represented by the following equilibrium: H2Ols) H2O() The AS° for this process should be O negative zero O positive
Chapter17: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 4ALQ: What types of experiments can be carried out to determine whether a reaction is spontaneous? Does...
Related questions
Question
Hand Written please.
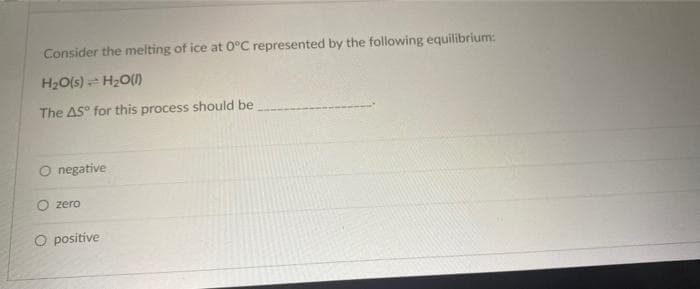
Transcribed Image Text:Consider the melting of ice at 0°C represented by the following equilibrium:
H2O(s) H20()
The AS° for this process should be
negative
O zero
O positive

Transcribed Image Text:Standard Gibb's free energy equation summarizes four thermodynamic properties: AG, AH", AS, and T. This
equation is very important to predict the spontaneity of a process. On your scratch paper, write out four different
situations that favor a spontaneous forward reaction. [You can use the "Kid @ Beach" analogy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning