SICI4 (I) Unbalanced equation 1. Given the following reaction: Si (s) + CI 2(g) 35g 56g ?g When 56g of silicon are combined with 35g of chlorine gas in a reaction vessel: Smce CI has the Smeallert value, wi be the a. What is the limiting reactant? Chtonne e liniting eachant b. How many moles of the excess reactant are left?
SICI4 (I) Unbalanced equation 1. Given the following reaction: Si (s) + CI 2(g) 35g 56g ?g When 56g of silicon are combined with 35g of chlorine gas in a reaction vessel: Smce CI has the Smeallert value, wi be the a. What is the limiting reactant? Chtonne e liniting eachant b. How many moles of the excess reactant are left?
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 100CP: he production capacity for acrylonitrile (C3H3N)in the United States is over 2 billion pounds per...
Related questions
Question
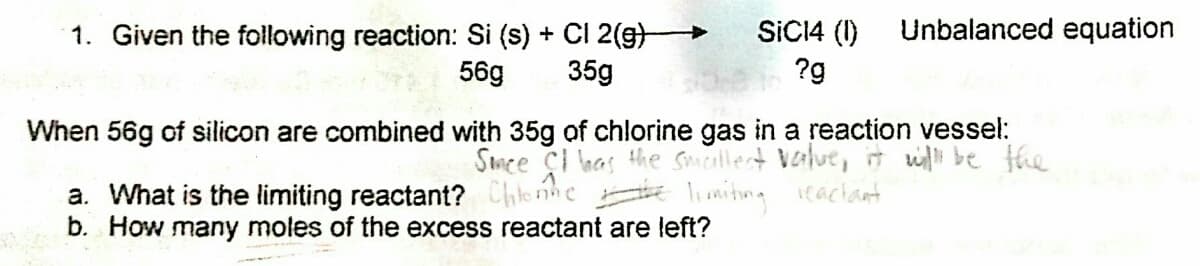
Transcribed Image Text:SICI4 (1)
Unbalanced equation
1. Given the following reaction: Si (s) + CI 2(g)
35g
56g
?g
When 56g of silicon are combined with 35g of chlorine gas in a reaction vessel:
Smee çI has the Smallect value,it wi be the
a. What is the limiting reactant? Chlonhe lniting eachant
b. How many moles of the excess reactant are left?

Transcribed Image Text:2. Take the reaction: NH3 + 02 → NO + H2O. In an experiment, 3.25g of NH3 are aliowed to
react with 3.50 g of O2.
a. Which reactant is limiting reagent?
b. How many grams of NO are formed?
c. How much of the excess reactant remains after the reaction?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning