Consider the neutralization reaction 2 HNO3(aq) + Ba(OH)2(aq) → 2 H2O(l) + Ba(NO3)2(aq) A 0.105 L sample of an unknown HNO3 solution required 41.1 mL of 0.200 M Ba(OH)2 for complete neutralization. What is the concentration of the HNO3 solution? concentration: Σ
Consider the neutralization reaction 2 HNO3(aq) + Ba(OH)2(aq) → 2 H2O(l) + Ba(NO3)2(aq) A 0.105 L sample of an unknown HNO3 solution required 41.1 mL of 0.200 M Ba(OH)2 for complete neutralization. What is the concentration of the HNO3 solution? concentration: Σ
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 151CP: Citric acid, which can be obtained from lemon juice, has the molecular formula C6H8O7. A 0.250-g...
Related questions
Question
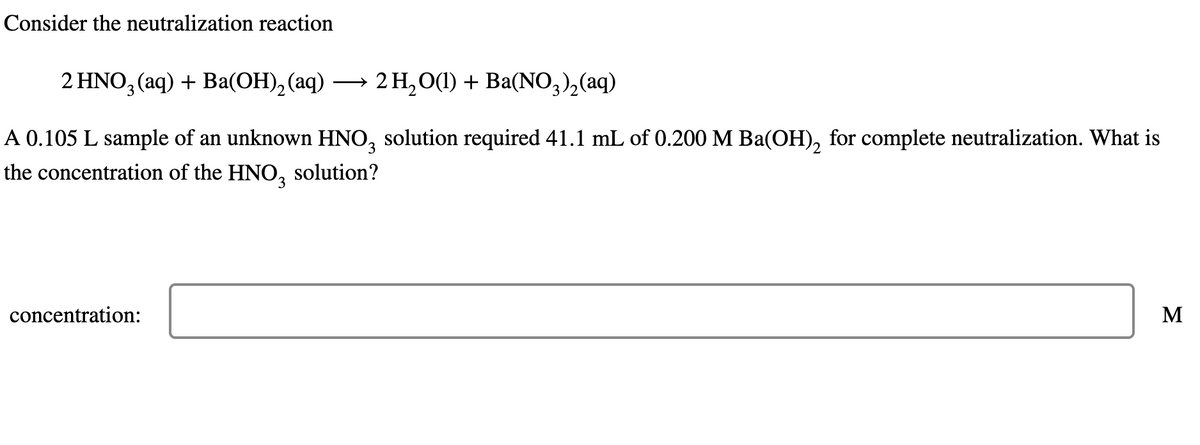
Transcribed Image Text:Consider the neutralization reaction
2 HNO3(aq) + Ba(OH)2(aq) →
2 H2O(l) + Ba(NO3)2(aq)
A 0.105 L sample of an unknown HNO3 solution required 41.1 mL of 0.200 M Ba(OH)2 for complete neutralization. What is
the concentration of the HNO3 solution?
concentration:
Σ
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning