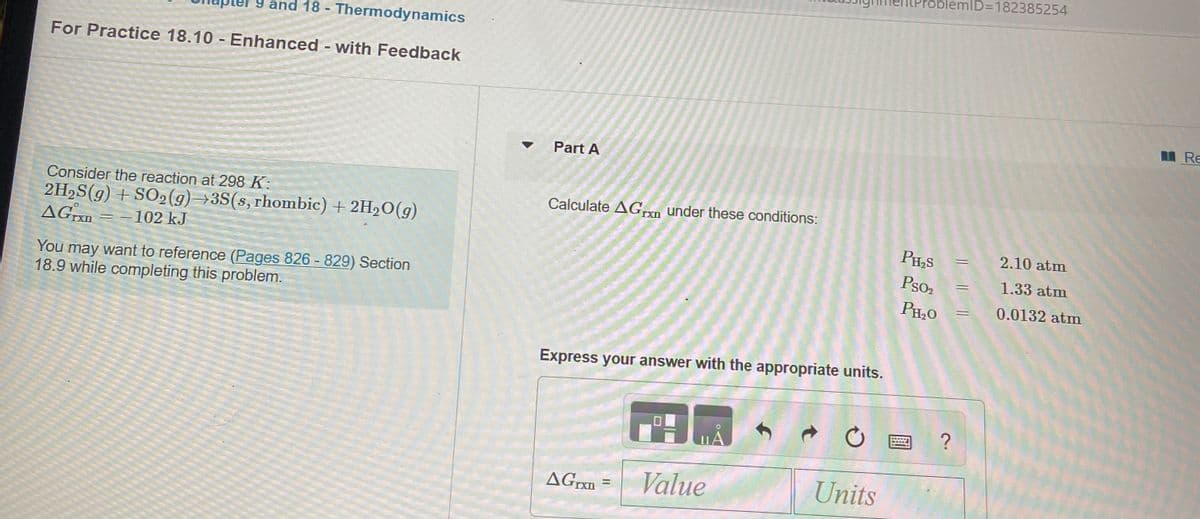
Consider the reaction at 298 K: 2H2S(g) + SO2(9) →3S(s, rhombic) + 2H2O(g) AGn = -102 kJ You may want to reference (Pages 826 - 829) Section 18.9 while completing this problem.
Q: 2) The thermal expansion coefficient is given by: a- 1(av vaT
A: If sample conc. is less than 1, there appears to be a second order PM-HO phase transition followed a...
Q: Chemistry 1 mole of ideal gas at 26.85 ⁰C that occupies 1 L of volume. Now this gas isothermally exp...
A: Here we have to determine entropy change of system , entropy change of surrounding and entropy chang...
Q: Suppose 1.97 g of iron() chioride (FeCl,) are dissolved in 890. ml of water. Find the composition of...
A: Relation between number of equivalents and number of moles is following. No. of equivalents = No. of...
Q: Activity3. True or False! Directions: Write T if the statement is correct and F if otherwise. Write ...
A: The statements have been explained in the following step.
Q: Q3: Determine the structure rules for Wurtzite (e.g. GaN) structure
A:
Q: 12 13 14 15 2.2 2.2 2 NIO,- 1.8 1.8 1.6- H1.6 1.4 1.4 1.2- NIÓ, 1.2 11 0.8 0.8 0.6 0.6 0.4 Ni** Ni,O...
A:
Q: Write the equation for the reaction of magnesium ions with sodium stearate. Explain this reaction us...
A: Sodium stearate formula is C17H35COONa. Magnesium stearate formula is (C17H35COO)2Mg.
Q: 4 What is the concentration of OAc- in a 0.4M Acetate buffer, pH 8? (pKa for Acetic acid is 4.77)
A:
Q: Take the mass measurement with nitrogen (28 dalton or atomic mass units per molecule) and the mass m...
A:
Q: The student realized that they did not dissolve all of the magnesium in the reaction and had to redo...
A:
Q: MnO, + + H+ Reactants Mn²+ + CO2 H2O Products 3
A:
Q: An arctic weather balloon is filled with 22.0 L of helium gas inside a prep shed. The temperature in...
A:
Q: An experiment involving the reaction between ammonium hydroxide and Cu2+ ions in aqueous solution wi...
A: In this investigation, understudies add alkali to an answer of copper(II) sulfate, notice the shadin...
Q: (k) When two proteins interact to form a bound complex, on average, what percentage of each protein'...
A: k) The covered surface region (BSA), which estimates the size of the point of interaction in a prote...
Q: Calculate the value of ΔGo in kJ for the combustion of 1 mole of methane to form carbon dioxide and ...
A: Thermodynamics is branch of chemistry in which deals with the amount of heat evolved or absorbed by ...
Q: In the presence of palladium in calcium carbonate, what is the IUPAC name of the product between the...
A: In the presence of palladium in calcium carbonate, what is the IUPAC name of the product between the...
Q: Chemistry A) can you predict the product of the reaction and show the mechanism that illustrates the...
A:
Q: 4-Aryl-5-tosyloxyhexanoates are converted to mixtures of lactones when exposed to silica or heated w...
A:
Q: Please answer the question below with complete solution:
A:
Q: What is the IUPAC name of the substance shown in the following model" ball & stick labels
A: Given,
Q: Single atoms only undergo electronic transitions. True False
A:
Q: Tetramethyl silane (TMS) is the only reference compound used to measure the chemical shifts of proto...
A: NMR spectroscopy is a very important tool for the determination of the structure of the organic comp...
Q: How can the relative supersaturation be varied during precipitate formation?
A: Ques: How can the relative supersaturation be varied during precipitate formation? Relative su...
Q: n one. Match enzyme or molecule with its function in bacteria. Answers may be used more than once or...
A: 37. RNA polymerase is the main enzyme in catalyzing DNA→ mRNA transcription. 38. DNA helicase unwin...
Q: The lon-product constant for water at a5C is 3.02x10 (a) What is the concentration of Hy0 ions at th...
A:
Q: e concepts of Intermolecular forces of attraction are important in ermining boiling point of a subst...
A:
Q: acylation. why does the synthesized compound exhibited fluorescence activity. and which layer in the...
A:
Q: Be sure to answer all parts. The reaction 2A B is second order with a rate constant of 51.0/M'min at...
A:
Q: A proportion of fentanyl is known to be lost to IV infusion bags made from PVC on storage and this e...
A:
Q: The vapor pressure of which of the following alkanes will be the highest? 2,3-dimethylpentane n-he...
A:
Q: Decide whether this proposed Lewis structure is reasonable. proposed Lewis structure Is the proposed...
A: The Lewis structure of a compound represents, basic structural representation of the molecule posit...
Q: Which one of the following compounds would adsorb most strongly to silica gel? SCH3 Br CO2H A B E
A: compound that would adsorb most strongly to silica gel is given below
Q: 23. The major product of the following reaction is (CH,CO),0 BF; Et,0, 0°C
A:
Q: 2NH2OH + Cu-N2H4 + Cu(OH)2 In the above redox reaction, use oxidation numbers to identify the elemen...
A: Oxidation is loss of electrons and Reduction is gain of electrons. Oxidizing agent is the species w...
Q: What is the major product of the reaction beloW! Draw a detailed plausible mechanism and all relevan...
A:
Q: monic 1876.06 cm1 for molecule N14 o16 is ,
A:
Q: Please give me the reagents and show the mechanism. Thank you so much.
A: These reaction involes the Michael addition reaction. Michael addition reaction involves the 1,4-add...
Q: (23) Convert these IUPAC formulas into skeletal structures. a. Cyclooctane b. trans-1,3-dimethylcycl...
A: Three questions based on nomenclature that are to be accomplished.
Q: Which acid in each pair has the stronger conjugate base? a. HI or HPO42−
A:
Q: sample of gelatin was treated with pepsin in the presence of HCI. After 15 min, the reaction wa with...
A:
Q: with a theoretical yield of 80% compute for the percent error of the following data Vial weight=...
A: Solution - Data given- theoretical yield= 80% resorcinol = 0.1502g Phthalic anhydride= 0.1022g
Q: Single atoms only undergo electronic transitions.
A:
Q: The energy requiring step during ATP synthesis by the FoF1-ATP synthase is The energy for this step ...
A: We have find out the answer.
Q: Calculate the [H3O+] of the following polyprotic acid solution: 0.360 M H3PO4. Calculate the pH of ...
A:
Q: a) What is the molarity of a solution made by dissolving 10 moles of sucrose, C12H22O11, in 10L of w...
A:
Q: If the temperature of the water at the end of the reaction was 22.0oC, what is the pressure of the w...
A: Given : Temperature = 22°C Pressure of water vapour is termed as vapour pressure of water.
Q: АСTIVITY 4 Name the following Hydrocarbons, by using the IUPAC Rules and its common name. A =PARENT ...
A: IUPAC nomenclature is based on 1)word root 2)suffix 3)prefix Rule- 1)Select parent chain or longest...
Q: acylation. why does the synthesized compound exhibited fluorescence activity. and which layer in the...
A:
Q: For the following unimolecular elimination reaction, draw the intermediate and the product(s) that w...
A: The first step of the given reaction involves the formation of carbocation. HCO3- acts as a base and...
Q: Which oil is more unsaturated and has the higher melting point, safflower oil or olive oil. Expound.
A: High oelic expeller pressed safflower oil has high monosaturated fat level . In fact it often has m...
Step by step
Solved in 2 steps with 2 images

- Thermodynamics Quantities for Selected Substances at 298.15 K (25⁰C) Substance ∆H⁰f (kJ/mol) ∆G⁰f (kJ/mol) S (J/K-mol) Carbon C2H2(g) 226.7 209.2 200.8 C2H4(g) 52.30 68.11 219.4 Hydrogen H2(g) 0 0 130.58 Oxygen O2(g) 0 0 205.0 H2O(l) -285.83 -237.13 69.91 1. What is the value of ∆S⁰ for the catalytic hydrogenation of ethene to ethane: C2H4(g) + H2(g) → C2H6(g) + 2H2O(l) in J/K?The table below provides data for the enthalpy and entropy of formation of compounds A and B at standard conditions (298 K) Compound DHfo (kJ mol-1) Sfo(J K-1mol-1) A –135.2 189.2 B –157.6 192.1 (b) A reversible isomerization reaction converts reactant A to product X. Calculate the Gibbs energy change at non-standard conditions (310 K) if the concentration of A is 2 x 10-4M and that of X is 3 x 10-6 M. Comment on whether the reaction is spontaneous at these conditions. The equilibrium constant Keq = 0.05. Assume standard temperature is 298K.The spontaneity of the following redox reaction with investigated by determining the change in Gibbs free energy at various temperature: Cu(s)+Ag^+(aq)--> Cu^2+(aq)+Ag(s) The data from the experiment is reproduced below: T/K DeltaG/kj 277.1 88.7665 287.1 82.2055 295.4 75.8375 304.9 69.0835 312.4 67.5397 322.5 64.8381 With the use of a spreadsheet program, generate a well-labeled plot of a DeltaG vs T and write down the equation of the trendline. From the graph, determine the deltaH and deltaS of the redox reaction. Explain the meaning of the changes in enthalpy and entropy of the reaction.
- Calculate the standard Gibbs reaction energy for the reaction at 298 K, 2CH3CHO(g) + O2(g) - > 2CH3 COOH(I) Given the following data, Sm\deg (CH3CHO(g )) = 250.30 J K-1 mol-1 Sm\deg (O2(g)) = 205.10 JK -1 mol-1 Sm\deg (CH3COOH(l))=159.80~K-1~mol-1\backslash Delta fHm\deg (CH3CHO(g))=-166.20~kJ~mol-1\backslash Delta fHm\deg (O2(g)) = 0.00 kJ mol-1 \Delta fHm\ deg (CH3COOH(I)) = 484.50 kJ mol-1The standard Gibbs energy of formation of gaseous ozone at 25.0 ℃ ΔfGo, is162.3 kJ. mol-1, for standard state of 1 bar. The reaction is: 3O2 (g) ⇌ 2O3(g). Calculate the value of Kx at 2 bar.For an aqueous solution saturated in both AgCl and AgI, at 1 bar and at 298 K. The equilibrium constants for this system are given as follow: Ksp(AgCl) = 1.8*10^-10, Ksp(AgI) = 8.5*10^-17, and Kw = 1.0*10^-14. How many formalities must be given to get unique values for all the concentrations?
- Reaction KspKsp ΔH°ΔH° ΔS°ΔS° FeCO3(s)⇄Fe2+(aq)+CO32−(aq)FeCO3(s)⇄Fe2+(aq)+CO32−(aq) 3×10−113×10−11 <0<0 >0>0 MnCO3(s)⇄Mn2+(aq)+CO32−(aq)MnCO3(s)⇄Mn2+(aq)+CO32−(aq) 2×10−112×10−11 <0<0 >0 The table above lists the equilibrium constants and changes in thermodynamic properties for the dissolution of FeCO3 and MnCO3 at 25°C. The two-particle diagrams below represent saturated solutions of each compound at equilibrium. (see attached image) a.) The particle diagrams best represent that ΔH°<0ΔH°<0 because the ions from both compounds are solvated by water molecules. b.) The particle diagrams best represent that ΔH°<0ΔH°<0 because both compounds produce about the same amount of CO32−CO32− ions from the dissolution. c.) The particle diagrams best represent that ΔS°>0ΔS°>0 because both compounds produce a very small amount of ions from the dissolution. d.) The particle diagrams best represent that the molar solubility is greater for…Thermodynamics Quantities for Selected Substances at 298.15 K (25⁰C) Substance ∆H⁰f (kJ/mol) ∆G⁰f (kJ/mol) S (J/K-mol) Hydrogen H2(g) 0 0 130.58 Oxygen O2(g) 0 0 205.0 H2O(l) -285.83 -237.13 69.91 10. What is the ∆S⁰ in the combustion of hydrogen in the presence of excess oxygen yields water: 2H2(g) + O2(g) → 2H2O(l) in J/K?A) Calculate the standard reaction entropy at 298K of 1) Zn(s)+___Cu(aq) _____Zn(aq)+Cu (s) 2) C12H22O11(s)+12O2(g)____12CO2(g) + 11H2O(l) B) Continue the reaction entropies above with the reaction enthalpies, and calculate the standard reaction Gibbs energy at 298K. C) Use standard Gibbs energies of formation to calculate the standard reaction Gibbs energies at 298K of the reactions above.
- The entropy of reaction at T=198.15K and a pressure of 5 bar.What is Ecell at 25°C for the reaction Mg(s) | Mg2+(2.347 M) || Sn2+(0.180 M) | Sn(s)? Report your answer to two decimal places and do not include units. Half-reaction E° (V) Mg2+ + 2e- → Mg(s) -2.37 Sn2+ + 2e- → Pb(s) -0.14Ca2+ and CO32- are dissolved in a beaker of sea water at 298 K, resulting in formation of a CaCO3precipitate:Ca2+ (aq) + CO32- (aq) ⇋ CaCO3 (s) ΔH0 = 13.44 kJ mol-1, ΔS0 = -120 J mol-1 K-1(i) Determine the direction of spontaneity under standard state conditions.(ii) Determine ΔG when [Ca2+] = 0.01 mol dm-3 and [CO32-] = 45 μmol dm-3. The activity coefficients of Ca2+ and CO32- in sea water are 0.28 and 0.21 respectively.

