Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 114AE: Consider the two space shuttle fuel reactions in Exercises 81 and 82. Which reaction produces more...
Related questions
Question
Transcribed Image Text:I Review | Constants | Periodic Table
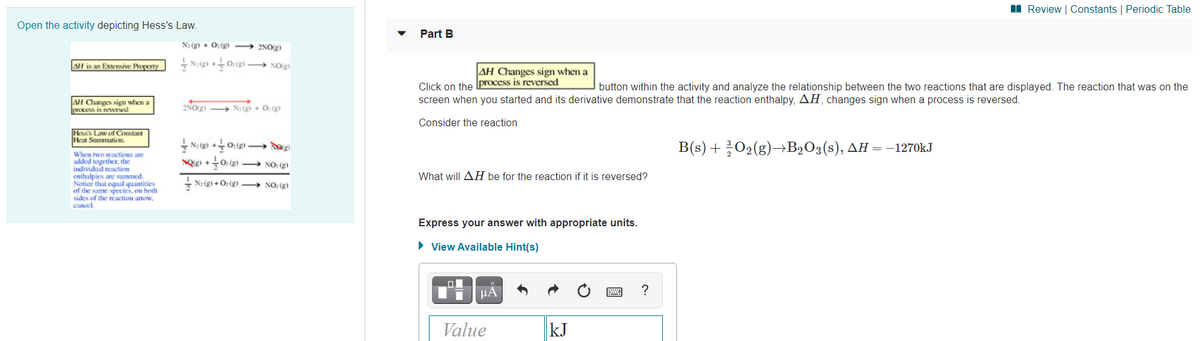
Open the activity depicting Hess's Law.
Part B
N: (g) + 0: (g)
2NOg)
AH is an Extensive Property
+ N: ) +0:() → NO(g)
AH Changes sign when a
Click on the process is reversed
screen when you started and its derivative demonstrate that the reaction enthalpy, AH, changes sign when a process is reversed.
button within the activity and analyze the relationship between the two reactions that are displayed. The reaction that was on the
AH Canges sign when a
process is reversed
2NO(g) → N:(gì + 0: (g)
Consider the reaction
Hess Law of Constant
icat Sumnation
+ N: (g) +0: (g) – d
NQ +0:()
B's) + 302(g)—B>0ҙ(s), дн — -1270KJ
When two reactions are
added together. the
individual reaction
enthalpies are summed.
Notice that equal quantities
of the same species, on both
sides of the reaction arrow,
cancel.
→ NO: g
What will AH be for the reaction if it is reversed?
+ N: (g) + O: (g → NO: (g)
Express your answer with appropriate units.
• View Available Hint(s)
µA
?
Value
kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning