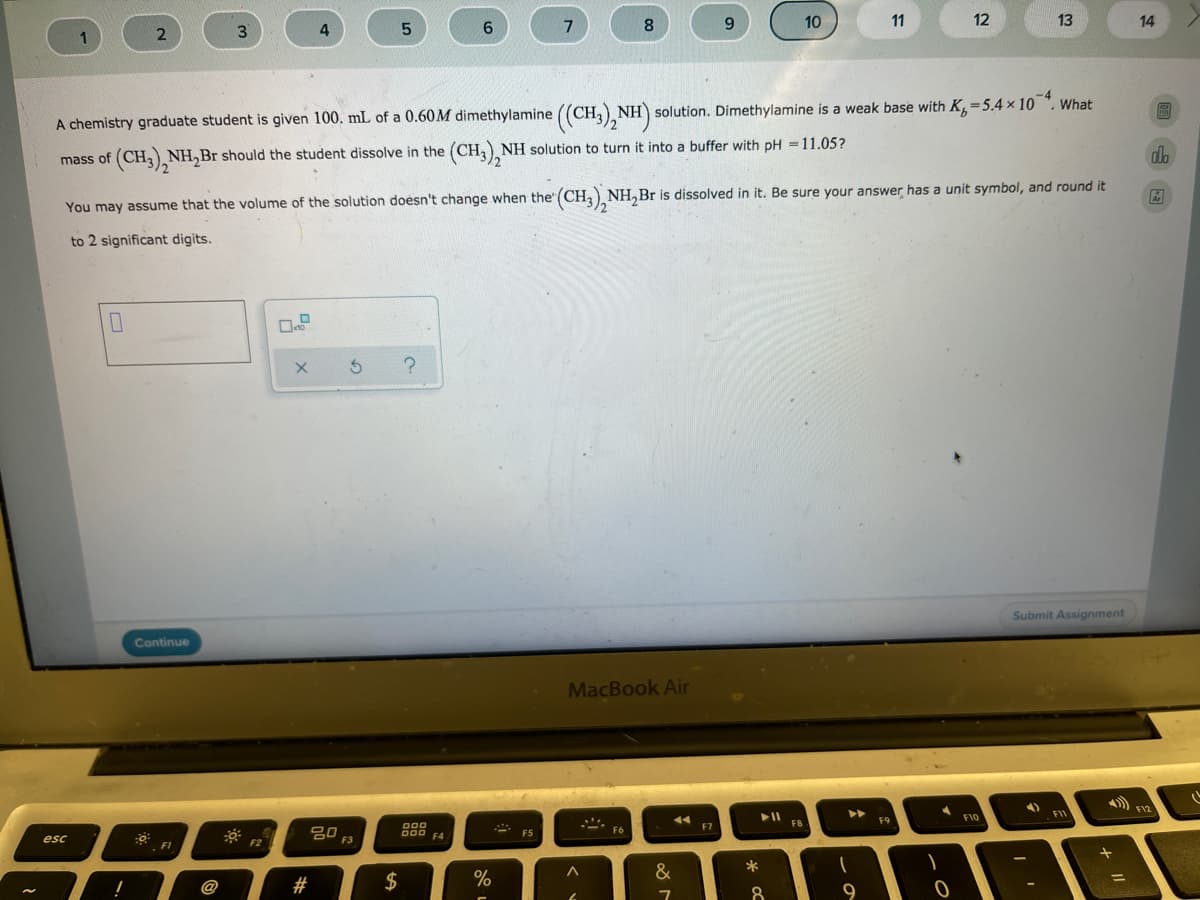
A chemistry graduate student is given 100. mL of a 0.60M dimethylamine ((CH) NH) solution. Dimethylamine is a weak base with K=5.4 × 10. What mass of (CH,) NH,Br should the student dissolve in the (CH,) NH solution to turn it into a buffer with pH =11.05? You may assume that the volume of the solution doesn't change when the" (CH,) NH,Br is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits.
A chemistry graduate student is given 100. mL of a 0.60M dimethylamine ((CH) NH) solution. Dimethylamine is a weak base with K=5.4 × 10. What mass of (CH,) NH,Br should the student dissolve in the (CH,) NH solution to turn it into a buffer with pH =11.05? You may assume that the volume of the solution doesn't change when the" (CH,) NH,Br is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 99AP
Related questions
Question
Transcribed Image Text:5.
6.
7
8
10
11
12
13
14
1
A chemistry graduate student is given 100. mL of a 0.60M dimethylamine ((CH,), NH) solution. Dimethylamine is a weak base with K,=5.4 × 10 ". What
mass of (CH,, NH,Br should the student dissolve in the (CH,), NH solution to turn it into a buffer with pH =11.05?
db
You may assume that the volume of the solution doesn't change when the" (CH, NH,Br is dissolved in it. Be sure your answer has a unit symbol, and round it
to 2 significant digits.
Submit Assignment
Continue
MacBook Air
F10
FI
F8
F9
000
F7
80
F6
D00 F4
F5
esc
F3
FI
*
@
#
$
9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
