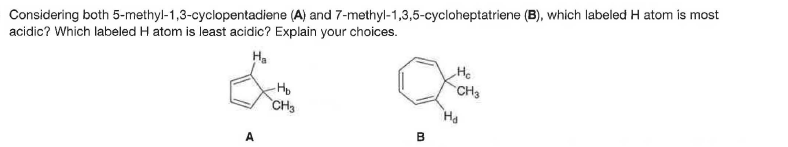
Considering both 5-methyl-1,3-cyclopentadiene (A) and 7-methyl-1,3,5-cycloheptatriene (B), which labeled H atom is most acidic? Which labeled H atom is least acidic? Explain your choices. Ha He CH3 CH3 A
Q: 2. What is the best name for the following molecule? a. (S)-4-methyl-3-oxo-7-octene b.…
A: While writing the IUPAC name first identify the parent name and the functional group.
Q: Be sure to answer all parts. Rank the following groups in order of decreasing priority: A. CH CH2 В.…
A: According to IUPAC the priority order should be: Hydrogen<Alkane<Alkyne<Alkene
Q: 10. Write priorities on these groups ( 1= highest, 4 = lowest) and assign the stereocenter as R or…
A: Here we are required to assign R /S configuration to stereocentre.
Q: Draw the major organic product of the reaction. Indicate the stereochemistry via wedge-and-dash…
A:
Q: Draw and name all stereoisomers of 3-methyl-2,4-hexadiene, using E-Z nomenclature
A: Stereoisomers are the compounds with same molecular formula but with different orientation in space.
Q: Draw 2,2 - dichloro - 3 - methylpentane . Fill in all H’s .
A: The given compound is: 2,2-dichloro-3-methylpentane. The parent carbon chain is pentane i.e. a…
Q: 1. Draw one of the two enantiomers of the major product from this 2. Draw 3. Draw the major product…
A:
Q: Arrange the following group in order of increasing priority. Q) -OCH3 -CH(CH3)2 -B(CH2CH3)2 -H
A: The CIP sequence rules are used to assign the R and S configuration. Rule 1: First, select the…
Q: What diene and dienophile are needed to prepare the following compound? Choose the single best…
A:
Q: Which structure is the best representation of a Zwitterion? a) b) H;N- H2N-CH-C- CH- -CH-C-OH…
A: Zwitterion is a molecule that is electrically neutral having both positive and negative charge on…
Q: в -OH -Br OCH CH3 -OCH2CH3 D Br
A:
Q: 2. Arrange these carbocations in order of increasing stability. CH2 CH3 -CH3 C. b. a.
A: Stability of carbocation can be compared using hyperconjugation effect. Carbocations with having…
Q: Taking into account anti periplanar geometry, predict the major E2 product formed from each starting…
A: Introduction : We have to tell the major eliminated product formed .
Q: Rank each group of radicals in order of increasing stability. (CH3),ĊCH,CH(CH3)2 (CH),CHCHCH(CH,)2…
A: a.) Carbon radicals are electron deficient so they tend to get stabilized by any factor which…
Q: Given that an E2 reaction proceeds with anti periplanar stereochemistry, draw the products of each…
A: Elimination reactions: Organic reactions contain the removal of a group of atoms from a molecule by…
Q: Which molecules A through D shown below would, upon hydrogenation of the double bond with Hz and…
A: Stereochemistry of organic compounds. Enantiomers: Enantiomers are non super impossible mirror…
Q: Draw all possible stereoisomers of hepta-2,4-diene and label each double bond as E or Z.
A: In the naming of geometric isomers, E, Z notation are used instead of cis, trans teams.Where, Z…
Q: Rank the set of substituents below in order of priority according to the Cahn-Ingold-Prelog sequence…
A:
Q: (a) Show how you would synthesize the pure (R) enantiomer of 2-butyl methyl sulfide, starting with…
A: The starting material in both the case is (R)-butan-2-ol. The structure is shown below:
Q: Which group in following pair is assigned the higher priority? −I, −Br
A: Iodine has the higher atomic number than bromine
Q: The compound cyclohex-3-en-1-one can give 2 carbanions. Point out the most stable and the reason?
A:
Q: Consider both 5-methyl-1,3-cyclopentadiene (A) and 7-methyl-1,3,5-cycloheptatriene (B). Ha Hc -Hp Ha…
A:
Q: Considering both 5-methylcyclopenta-1,3-diene (A) and 7-methylcyclohepta-1,3,5-triene (B), which…
A: Acid: According to Bronsted theory, the substance which can easily donate its proton is called…
Q: Owing from the most to the least stable carbocation. B) Sectio A) O CH: C) D) E) CH, CH,CH CH,CH;-…
A: Question 1 order of stability of carbocation A > B > C > E > D A compound is benzyl…
Q: 4. Rank the following leaving groups from best to worst. OH2 -OH -CH3 -NH2 LG LG LG LG LG A B C D E
A: Ability to leave of a leaving group depends upon: Electronegativity of the leaving group: A high…
Q: Considering both 5-methylcyclopenta-1,3-diene (A) and 7methylcyclohepta-1,3,5-triene (B), which…
A:
Q: 6. a) For each of the following pairs of molecules, identify the stereochemical relationship. Are…
A:
Q: Considering both 5-methylcyclopenta-1,3-diene (A) and 7-methylcyclohepta-1,3,5-triene (B), which…
A: On losing Hb, the compound gain aromaticity. Thus, Hb will be the most acidic, and Ha will be the…
Q: HX (1 equiv), cold (7) Conjugated diene 1,2-Adduct H. (8) HX (1 equiv), warm C. Conjugated diene…
A: Addition of HX to conjugated alkenes occurs via two modes: Direct addition (1,2 addition): HX adds…
Q: What are the reagents A and B in the scheme below? CH, CH,CH,CH,C-CH, ČI в A H,C CH H,C CH,
A:
Q: isopentane 4-methylcyclopent-1-ene toluene
A: Here we have to determine the compounds that can reacts with I2/KI and that can reacts with Br2…
Q: Be sure to answer all parts. Rank the following groups in order of decreasing priority: A. CH CH, B.…
A:
Q: Which reaction below follows Markovnikov's rule? А. CH;CH,CH=CH2 CH3CH2CH2CH2 ČI CH; B.…
A:
Q: H₂C H Br CH3 is what configuration? a. 1. b. S What is the best leaving group? F- a. 2. b. Cl- Br-…
A: In a nucleophilic reaction, the incoming electron-rich moiety attacks the electron-deficient carbon…
Q: 4-bromo-3-ethyl-5-methylcyclohexanone H;C-H2C- b) H;C- а) Br Br ČH2-CH3 ČH3 H;C-H2C- d) Br c) Br CH3…
A:
Q: Considering both 5-methylcyclopenta-1,3-diene (A) and 7-methylcyclohepta-1,3,5-triene (B), which…
A: In case of A, if we remove the Hb+ then we will have a -ve charge on carbon which is in conjugation…
Q: Rank the following carbocations from least to most stable: 1 2 O1<3<2 O 2<1< 3 O1<2<3 O 2<3 <1
A: Carbocation stability depends upon the number of alkyl groups attached to it. The alkyl groups have…
Q: Which of the following is the most effective way to stabilize carbocations? a. Zaitzev's rule O b.…
A: Carbocation stablized by electron donating group or resonance when positive charge is in conjugation…
Q: A)Circle all of the stereo centers in MDMA. B) assign the absolute stereochemistry (R or S) for each…
A:
Q: b) CH3-CH-CH-CH; ОН ОН frpm CH3-CHOH-CH,-CH3
A:
Q: 5. Rank the following groups in order of increasing leaving group ability (i.e. rank from worst LG…
A: Order of increasing leaving group ability:
Q: Considering both 5-methylcyclopenta-1,3-diene (A) and 7-methylcyclohepta-1,3,5-triene (B), which…
A: In case of A, if we remove the Hb+ than we will have a -ve charge on carbon which is in conjugation…
Q: Bicyclo[2.2.1]heptan-7-one + PCC (in CH₂Cl₂) => A.) Bicyclo[2.2.1]heptan-7-ol B.)…
A: PCC oxidizes alcohols . From primary alcohols to aldehydes and from secondary alcohols to ketones.…
Q: predict the major product of the reaction sequence below
A:
Q: For each pair below, briefly explain and illustrate why the structure circled is most stable.…
A: A question based on introduction to organic chemistry that is to be accomplished.
Q: What is the product of the following reaction? H3C-CH,-CH=CH2 HBr JF-MO5 H;C-CH-CH2-CH3 Br JF-MO5а…
A: Anti Markovnikov rule portrays the regiochemistry in which the substituent is clung to a less subbed…
Q: Rank the following substituents in order of increasing activation strength: a. -N(CH3)2 b. -CN С.…
A:
Q: Why is - CN a weaker leaving group than - NHCH3.
A: Weak Bases are the Best Leaving Groups. (Recall that the stronger the acid, the weaker the conjugate…
Q: 7. For each reaction, circle the correct product. Br N2OCH3 or NaOCH3 or E2 E2
A: A species with a larger size can easily accommodate negative charge. In other words, in a large size…
Q: Identify compounds B-G according to the following synthetic scheme. CH₂Br A Mg ether B D D₂O 1.…
A: Products B-G formed as follows,
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- Considering both 5-methylcyclopenta-1,3-diene (A) and 7methylcyclohepta-1,3,5-triene (B), which labeled H atom is most acidic? Which labeled H atom is least acidic? Explain your choices.Considering both 5-methylcyclopenta-1,3-diene (A) and 7-methylcyclohepta-1,3,5-triene (B), which labeled H atom is most acidic?Which labeled H atom is least acidic? Explain your choices.a.Draw three-dimensional representations for all stereoisomers of 2chloro-3-methylpentane, and label pairs of enantiomers. b. Considering dehydrohalogenation across only C2 and C3, draw the E2 product that results from each of these alkyl halides. How many different products have you drawn? c. How are these products related to each other?
- Please fill out for each reaction i.Fill in the missing starting materials, products, or reagents as necessary.If no reaction occurs, write "N.R." and explain why this is the case. ii. Label each transformation as SN1, SN2, or acid/base. iii. Indicate if the product is racemic or a single enantiomer.a. Draw three-dimensional representations for all stereoisomers of 2-chloro-3- methylpentane, and label pairs of enantiomers.b. Considering dehydrohalogenation across C2 and C3 only, draw the E2 product that results from each of these alkyl halides. How many different products have you drawn?c. How are these products related to each other?The 1,2‑dibromide is synthesized from an alkene starting material. Draw the alkene starting material. Clearly, show stereochemistry of the alkene.
- Draw and name all stereoisomers of 3-chlorohepta-2,4-diene using the E-Z nomenclature.Can someone please explain the logic of 2 vs 3 for stereochemistry? They are both C-C so I don't understand how to rank 2 vs 3. Thank you!Arrange the following group in order of increasing priority. Q) -OCH3 -CH(CH3)2 -B(CH2CH3)2 -H
- the answer is C. Why is it an eliination reaction vs a SN2 reaction? I would think the answer is BDraw and name all stereoisomers of 3-chlorohepta-2,4-diene(a) using the cis-trans nomenclature.(b) using the E-Z nomenclatureShow how HC≡CH, CH3CH2Br, and (CH3)2CHCH2CH2Br can be used to prepare CH3CH2C≡CCH2CH2CH(CH3)2. Show all reagents, and use curved arrows to show movement of electron pairs.

