Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
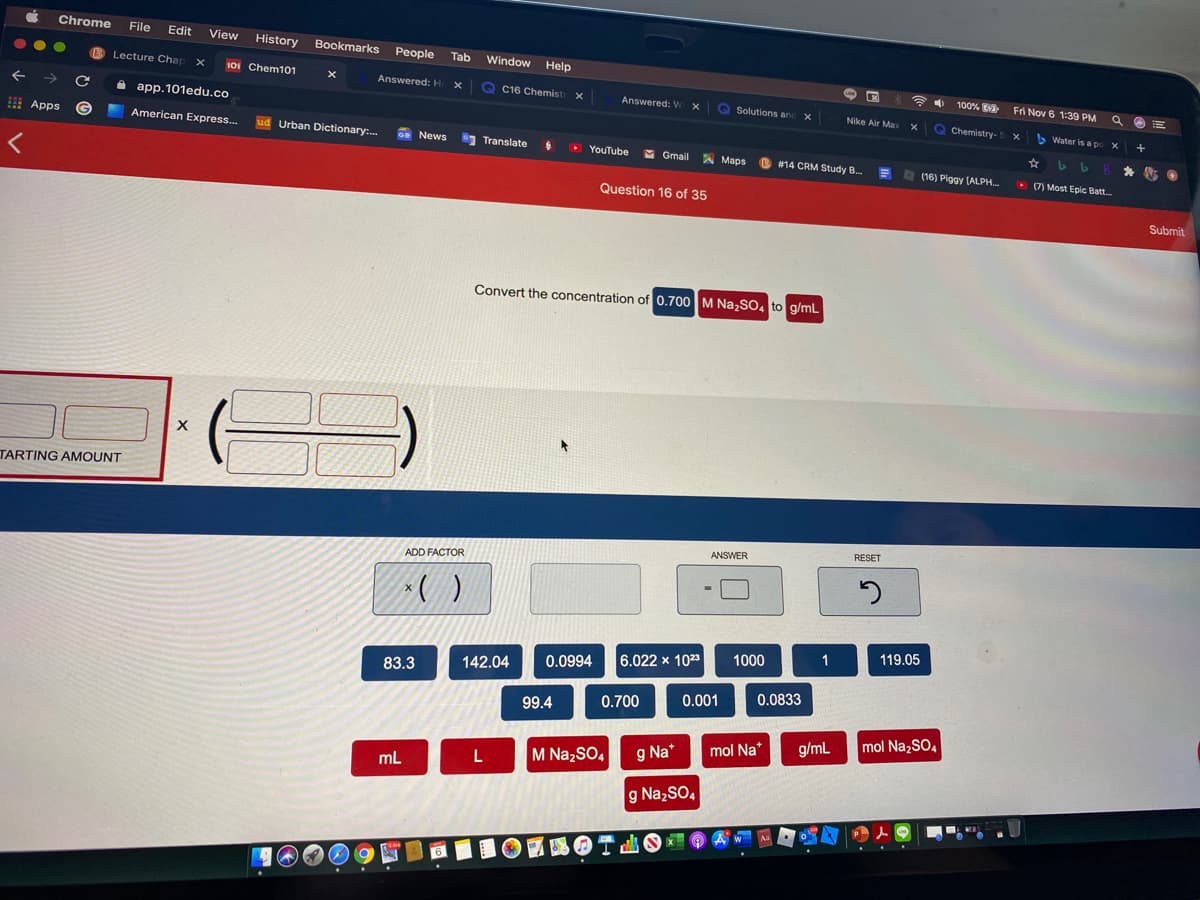
Problem 9.90PAE: 9.90 Many engineering designs must incorporate ways to dissipate energy in the form of heat. Water...
Related questions
Question
100%
Transcribed Image Text:Chrome
File
Edit
View
History
Bookmarks
People
Tab
Window
Help
B Lecture Chap x
101 Chem101
Answered: H
O c16 Chemistr x
100% Ea
Fri Nov 6 1:39 PM
Answered: W
Solutions anc X
A app.101edu.co
Nike Air Max
O Chemistry-
Water is a po x
: Apps
American Express..
ud Urban Dictionary:.
☆ b b
OE News
Translate
O YouTube
M Gmail
A Maps
B #14 CRM Study B.
(16) Piggy (ALPH.
(7) Most Epic Batt.
Question 16 of 35
Submit
Convert the concentration of 0.700 M Na,So, to g/mL
TARTING AMOUNT
RESET
ANSWER
ADD FACTOR
1
119.05
6.022 x 1023
1000
142.04
0.0994
83.3
0.001
0.0833
99.4
0.700
g/mL
mol NazSO4
g Na*
mol Na*
L
M Na,SO4
mL
g NazSO,
@T山
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning