Cps//igital.wwnorton.com/34868 Joresight Bank Imported From IE 24% kelly mehrkens@mnsu edu mework#5 02/16/20 20,H10 130,- SCo,+10H,0 7th attempt Q See Hint See Periodic Table If the combustion of 45.24 g of C4H10 produces 100.11gof CO2. What is the percent yield of the reaction? (Assume oxygen is in excess.) 28.44 6th attempt 5th attempt < 07/18 SURMIL ANSKER VIEW SOLUTION 4OF 18 OUESTIONS COMPLETED ext Smartworks Googl..
Cps//igital.wwnorton.com/34868 Joresight Bank Imported From IE 24% kelly mehrkens@mnsu edu mework#5 02/16/20 20,H10 130,- SCo,+10H,0 7th attempt Q See Hint See Periodic Table If the combustion of 45.24 g of C4H10 produces 100.11gof CO2. What is the percent yield of the reaction? (Assume oxygen is in excess.) 28.44 6th attempt 5th attempt < 07/18 SURMIL ANSKER VIEW SOLUTION 4OF 18 OUESTIONS COMPLETED ext Smartworks Googl..
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter11: Stoichiometry
Section: Chapter Questions
Problem 90A
Related questions
Question
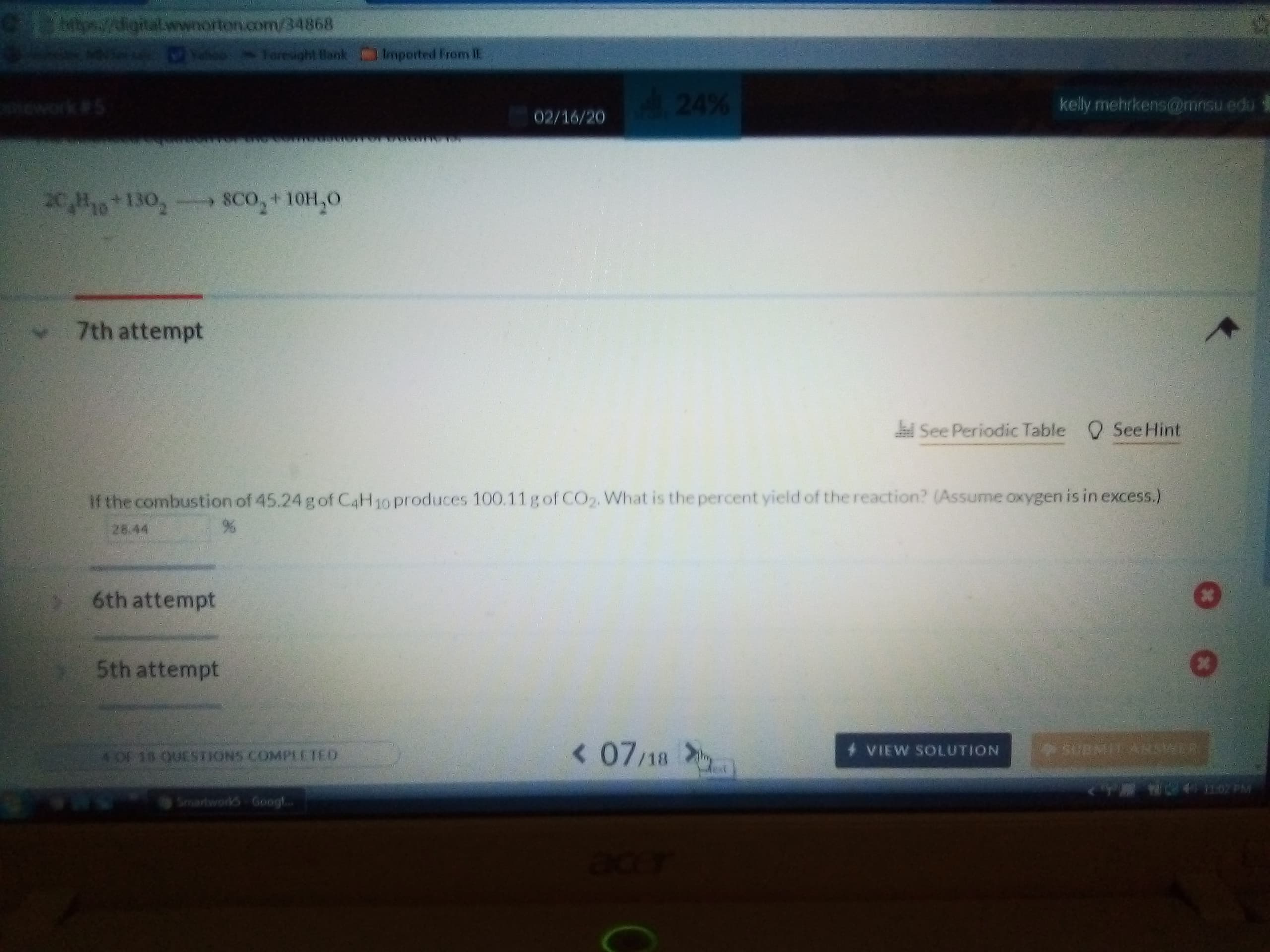
Transcribed Image Text:Cps//igital.wwnorton.com/34868
Joresight Bank Imported From IE
24%
kelly mehrkens@mnsu edu
mework#5
02/16/20
20,H10 130,- SCo,+10H,0
7th attempt
Q See Hint
See Periodic Table
If the combustion of 45.24 g of C4H10 produces 100.11gof CO2. What is the percent yield of the reaction? (Assume oxygen is in excess.)
28.44
6th attempt
5th attempt
< 07/18
SURMIL ANSKER
VIEW SOLUTION
4OF 18 OUESTIONS COMPLETED
ext
Smartworks Googl..
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning