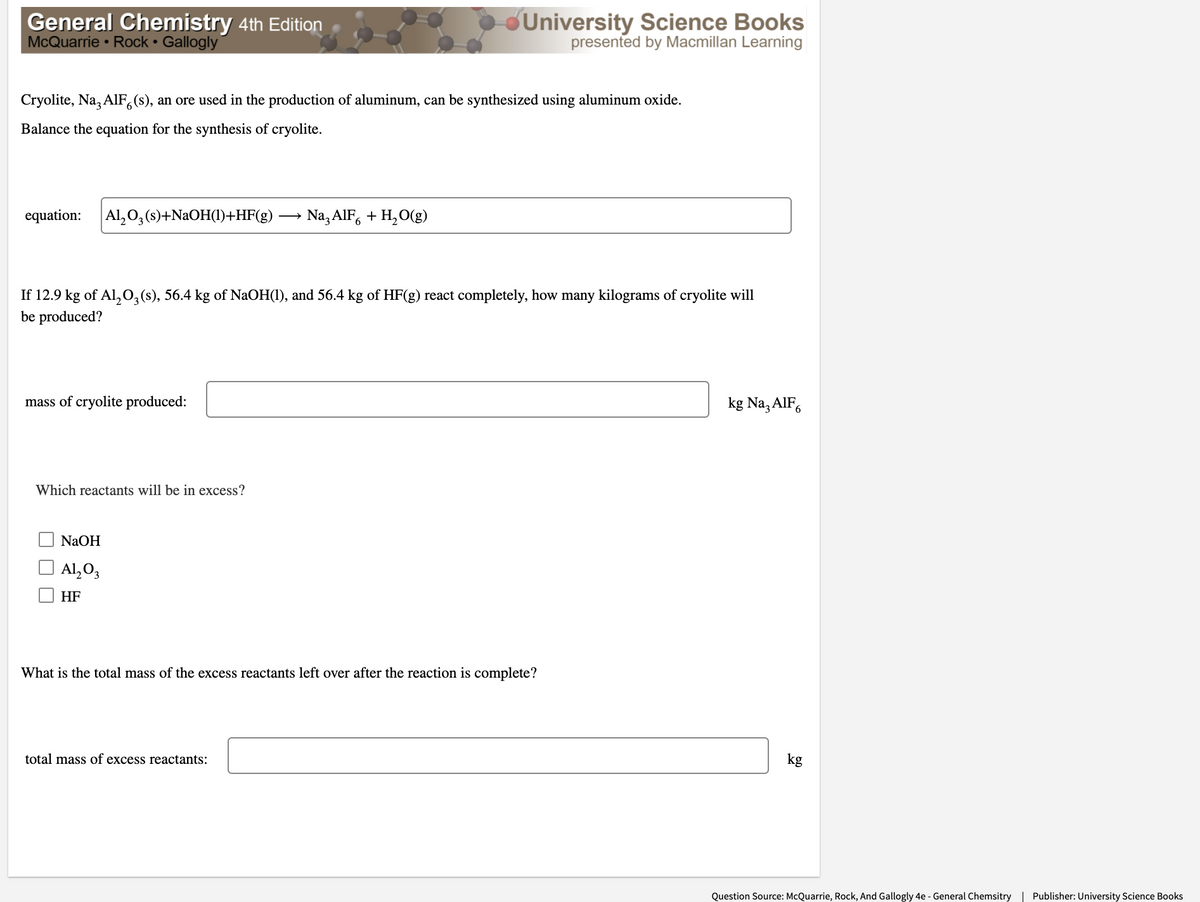
Cryolite, Na, AIF, (s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. Balance the equation for the synthesis of cryolite. equation: Al,0,(s)+NAOH(1)+HF(g) → Na, AIF, + H,O(g) If 12.9 kg of Al,0,(s), 56.4 kg of NaOH(1), and 56.4 kg of HF(g) react completely, how many kilograms of cryolite will be produced? mass of cryolite produced: kg Na, AIF, Which reactants will be in excess? NaOH Al,0, HF What is the total mass of the excess reactants left over after the reaction is complete? total mass of excess reactants: kg
Cryolite, Na, AIF, (s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. Balance the equation for the synthesis of cryolite. equation: Al,0,(s)+NAOH(1)+HF(g) → Na, AIF, + H,O(g) If 12.9 kg of Al,0,(s), 56.4 kg of NaOH(1), and 56.4 kg of HF(g) react completely, how many kilograms of cryolite will be produced? mass of cryolite produced: kg Na, AIF, Which reactants will be in excess? NaOH Al,0, HF What is the total mass of the excess reactants left over after the reaction is complete? total mass of excess reactants: kg
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter10: Fuels, Organic Chemicals, And Polymers
Section: Chapter Questions
Problem 89QRT
Related questions
Question
Transcribed Image Text:General Chemistry 4th Edition
McQuarrie • Rock • Gallogly
University Science Books
presented by Macmillan Learning
Cryolite, Na, AIF,(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide.
Balance the equation for the synthesis of cryolite.
equation:
Al, 0, (s)+NAOH(1)+HF(g)
Na, AIF, + H, O(g)
If 12.9 kg of Al,0,(s), 56.4 kg of NaOH(1), and 56.4 kg of HF(g) react completely, how many kilograms of cryolite will
be produced?
mass of cryolite produced:
kg Na, AlF,
Which reactants will be in excess?
NaOH
Al, O3
HF
What is the total mass of the excess reactants left over after the reaction is complete?
total mass of excess reactants:
kg
Question Source: McQuarrie, Rock, And Gallogly 4e - General Chemsitry | Publisher: University Science Books
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
