(d) ammonium carbonate and hydrochloric acid (e) potassium phosphate and calcium iodide (f) lead(II)nitrate and sulfuric acid (h) silver nitrate and lithium chloride Go 37 Cat icn 38 an icnS- negative Hydroxide Iodide Nitrate Phosphate Sulfate Sulfide Acetate Bromide | Carbonate Chloride НРО 2- Aluminum Sulfite aq aq --- - Ammonium aq aq aq aq aq Barium aq aq aq aq aq aq aq aq aq aq SS aq aq p. Calcium aq aq aq SS aq aq Copper(II) aq aq aq aq --- aq aq d aq Н.О() aq aq aq Iron(II) aq aq aq aq aq aq Iron(III) aq aq aq aq p. Lead(II) aq aq Magnesium Postvc Hg aq aq aq aq aq aq d. 2+ d. p. p. Hg,(PO,), p, HgO p. aq HgS --- Potassium aq aq aq aq aq aq aq aq aq aq aq aq Silver р. РОЗ- aq Sodium aq aq aq aq aq aq aq aq aq aq aq aq Zinc P, PO,- aq aq aq aq aq aq Key: aq aqueous (soluble in water); ss= slightly soluble in water (solid precipitate might be observed): p-precipitate in water (solid); W3D w weak electrolyte that is soluble in water (aq); d= decomposes in water
(d) ammonium carbonate and hydrochloric acid (e) potassium phosphate and calcium iodide (f) lead(II)nitrate and sulfuric acid (h) silver nitrate and lithium chloride Go 37 Cat icn 38 an icnS- negative Hydroxide Iodide Nitrate Phosphate Sulfate Sulfide Acetate Bromide | Carbonate Chloride НРО 2- Aluminum Sulfite aq aq --- - Ammonium aq aq aq aq aq Barium aq aq aq aq aq aq aq aq aq aq SS aq aq p. Calcium aq aq aq SS aq aq Copper(II) aq aq aq aq --- aq aq d aq Н.О() aq aq aq Iron(II) aq aq aq aq aq aq Iron(III) aq aq aq aq p. Lead(II) aq aq Magnesium Postvc Hg aq aq aq aq aq aq d. 2+ d. p. p. Hg,(PO,), p, HgO p. aq HgS --- Potassium aq aq aq aq aq aq aq aq aq aq aq aq Silver р. РОЗ- aq Sodium aq aq aq aq aq aq aq aq aq aq aq aq Zinc P, PO,- aq aq aq aq aq aq Key: aq aqueous (soluble in water); ss= slightly soluble in water (solid precipitate might be observed): p-precipitate in water (solid); W3D w weak electrolyte that is soluble in water (aq); d= decomposes in water
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter4: Nomenclature
Section: Chapter Questions
Problem 16A
Related questions
Question
D-H

Transcribed Image Text:(d) ammonium carbonate and hydrochloric acid
(e) potassium phosphate and calcium iodide
(f) lead(II)nitrate and sulfuric acid
(h) silver nitrate and lithium chloride
Go
37
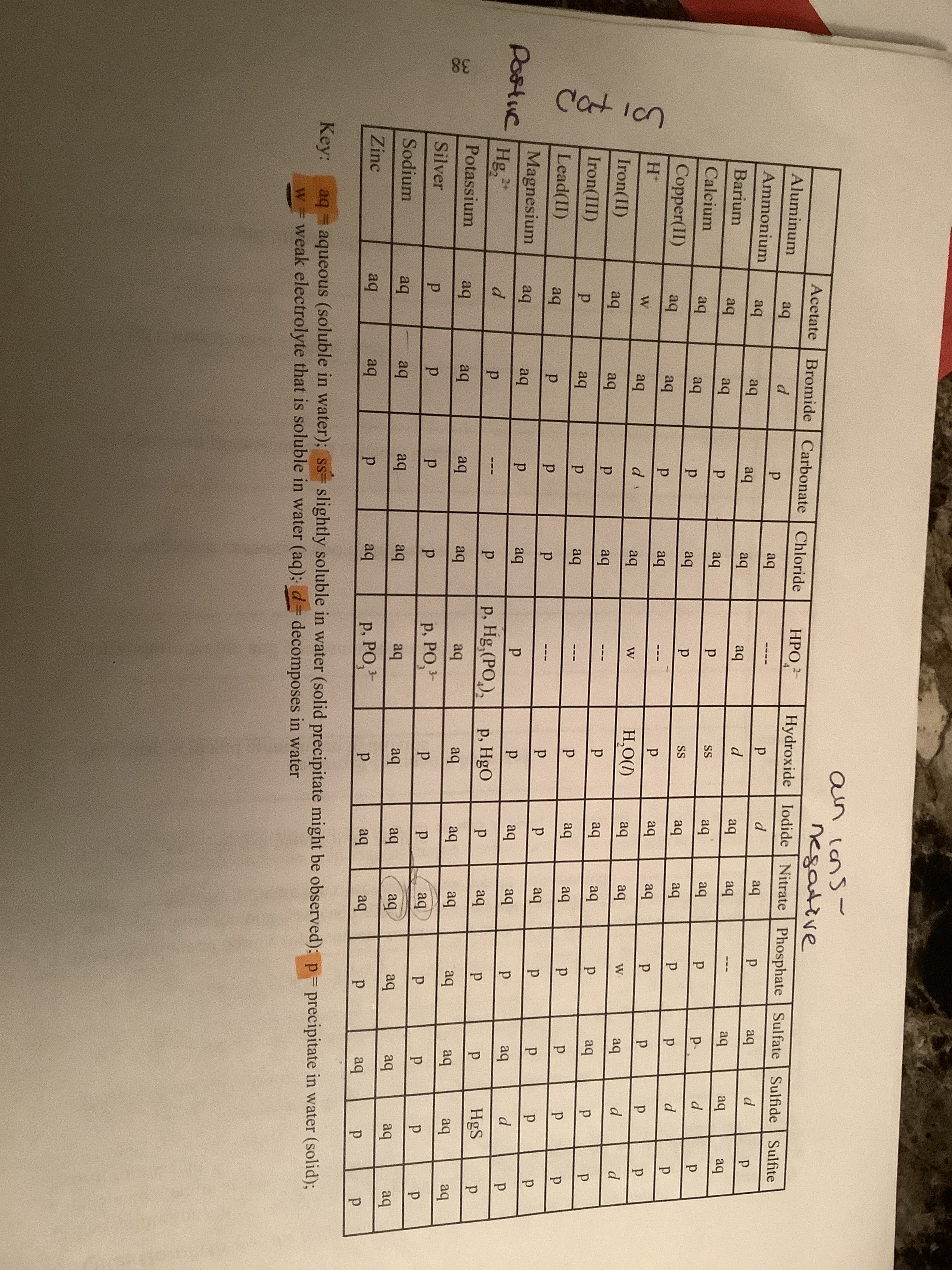
Transcribed Image Text:Cat icn
38
an icnS-
negative
Hydroxide Iodide Nitrate Phosphate Sulfate Sulfide
Acetate Bromide | Carbonate Chloride
НРО 2-
Aluminum
Sulfite
aq
aq
--- -
Ammonium
aq
aq
aq
aq
aq
Barium
aq
aq
aq
aq
aq
aq
aq
aq
aq
aq
SS
aq
aq
p.
Calcium
aq
aq
aq
SS
aq
aq
Copper(II)
aq
aq
aq
aq
---
aq
aq
d
aq
Н.О()
aq
aq
aq
Iron(II)
aq
aq
aq
aq
aq
aq
Iron(III)
aq
aq
aq
aq
p.
Lead(II)
aq
aq
Magnesium
Postvc Hg
aq
aq
aq
aq
aq
aq
d.
2+
d.
p.
p. Hg,(PO,),
p, HgO
p.
aq
HgS
---
Potassium
aq
aq
aq
aq
aq
aq
aq
aq
aq
aq
aq
aq
Silver
р. РОЗ-
aq
Sodium
aq
aq
aq
aq
aq
aq
aq
aq
aq
aq
aq
aq
Zinc
P, PO,-
aq
aq
aq
aq
aq
aq
Key:
aq aqueous (soluble in water); ss= slightly soluble in water (solid precipitate might be observed): p-precipitate in water (solid);
W3D
w weak electrolyte that is soluble in water (aq); d= decomposes in water
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax
