Use the periodic table to find the two ions (positive ion and negative ion) for each compound. Then write the formula. Remember, the overall charge of the compound is neutral (zero charge). Thus, the overall positive charge must be equal to the overall negative charge. Example: calcium chloride 2+ Ca C (These two charges are not the same, but 2 chloride ions will give an overali negative charge of -2. The positive ion already has a + 2 charge.) Ca²+ Cl₂ Therefore, CaCl₂ is the answer. Name 1) sodium chloride ex. 2) potassium bromide 3) calcium oxide 4) magnesium sulfide Ca²+ CI CI Positive Ion Na+ Count up the total charge. +2, -1, -1 This adds up to zero!! Negative Ion CI Formula NaCl K Br
Use the periodic table to find the two ions (positive ion and negative ion) for each compound. Then write the formula. Remember, the overall charge of the compound is neutral (zero charge). Thus, the overall positive charge must be equal to the overall negative charge. Example: calcium chloride 2+ Ca C (These two charges are not the same, but 2 chloride ions will give an overali negative charge of -2. The positive ion already has a + 2 charge.) Ca²+ Cl₂ Therefore, CaCl₂ is the answer. Name 1) sodium chloride ex. 2) potassium bromide 3) calcium oxide 4) magnesium sulfide Ca²+ CI CI Positive Ion Na+ Count up the total charge. +2, -1, -1 This adds up to zero!! Negative Ion CI Formula NaCl K Br
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter2: Atoms, Molescules, And Ions
Section: Chapter Questions
Problem 2.30QP
Related questions
Question
I'm having trouble understanding how to put these formulas together
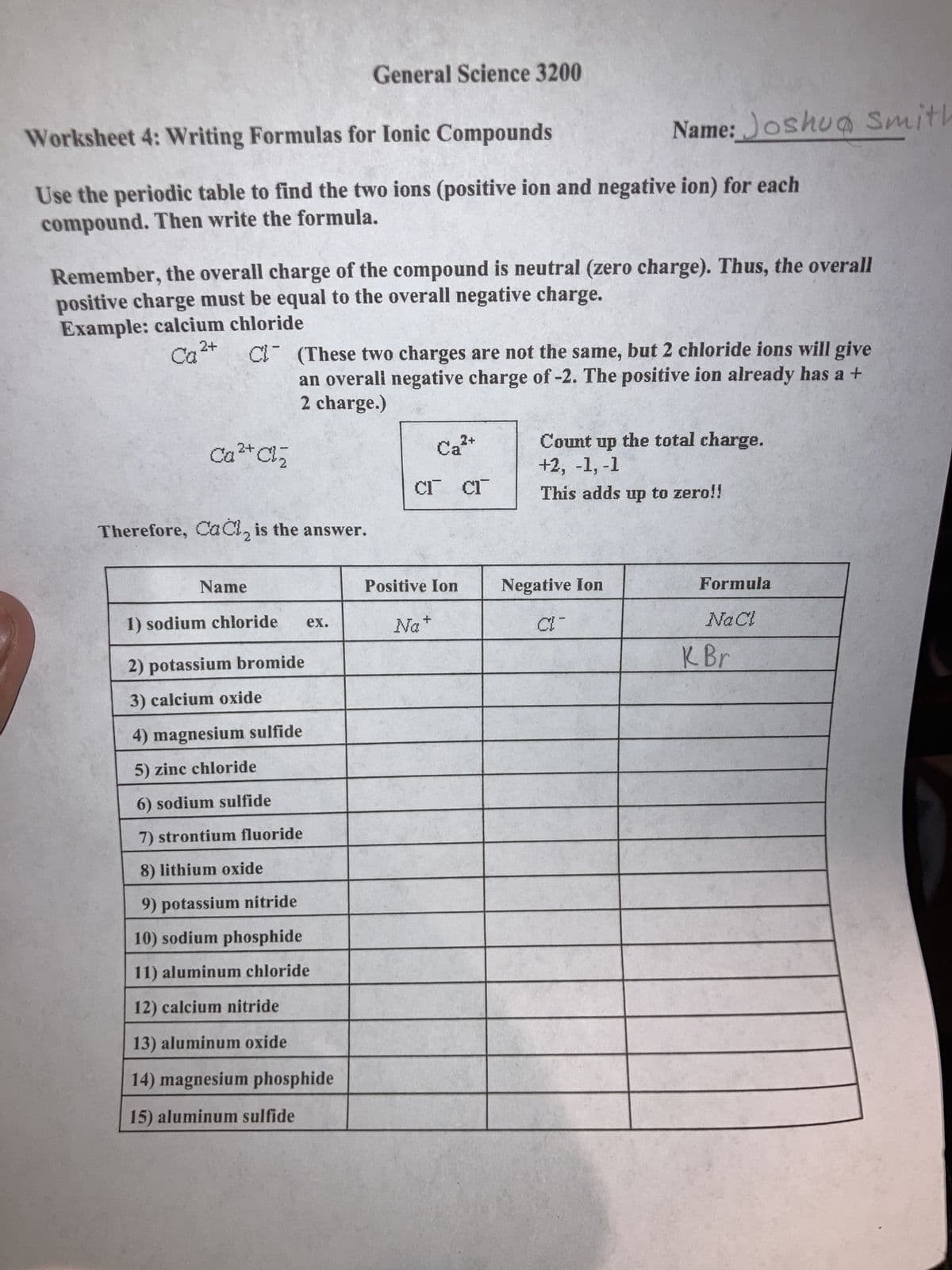
Transcribed Image Text:Worksheet 4: Writing Formulas for Ionic Compounds
Use the periodic table to find the two ions (positive ion and negative ion) for each
compound. Then write the formula.
Example: calcium chloride
CQ ²+
Remember, the overall charge of the compound is neutral (zero charge). Thus, the overall
positive charge must be equal to the overall negative charge.
2+
Ca²+ Cl₂
Therefore, CaCl₂ is the answer.
Name
C (These two charges are not the same, but 2 chloride ions will give
an overall negative charge of -2. The positive ion already has a +
2 charge.)
1) sodium chloride
2) potassium bromide
3) calcium oxide
General Science 3200
4) magnesium sulfide
5) zinc chloride
6) sodium sulfide
7) strontium fluoride
lithium oxide
ex.
9) potassium nitride
10) sodium phosphide
11) aluminum chloride
12) calcium nitride
13) aluminum oxide
14) magnesium phosphide
15) aluminum sulfide
Ca²+
CI CI
Name: Joshua Smith
Positive Ion
Na+
Count up the total charge.
+2, -1, -1
This adds up to zero!!
Negative Ion
CI-
Formula
NaCl
K Br
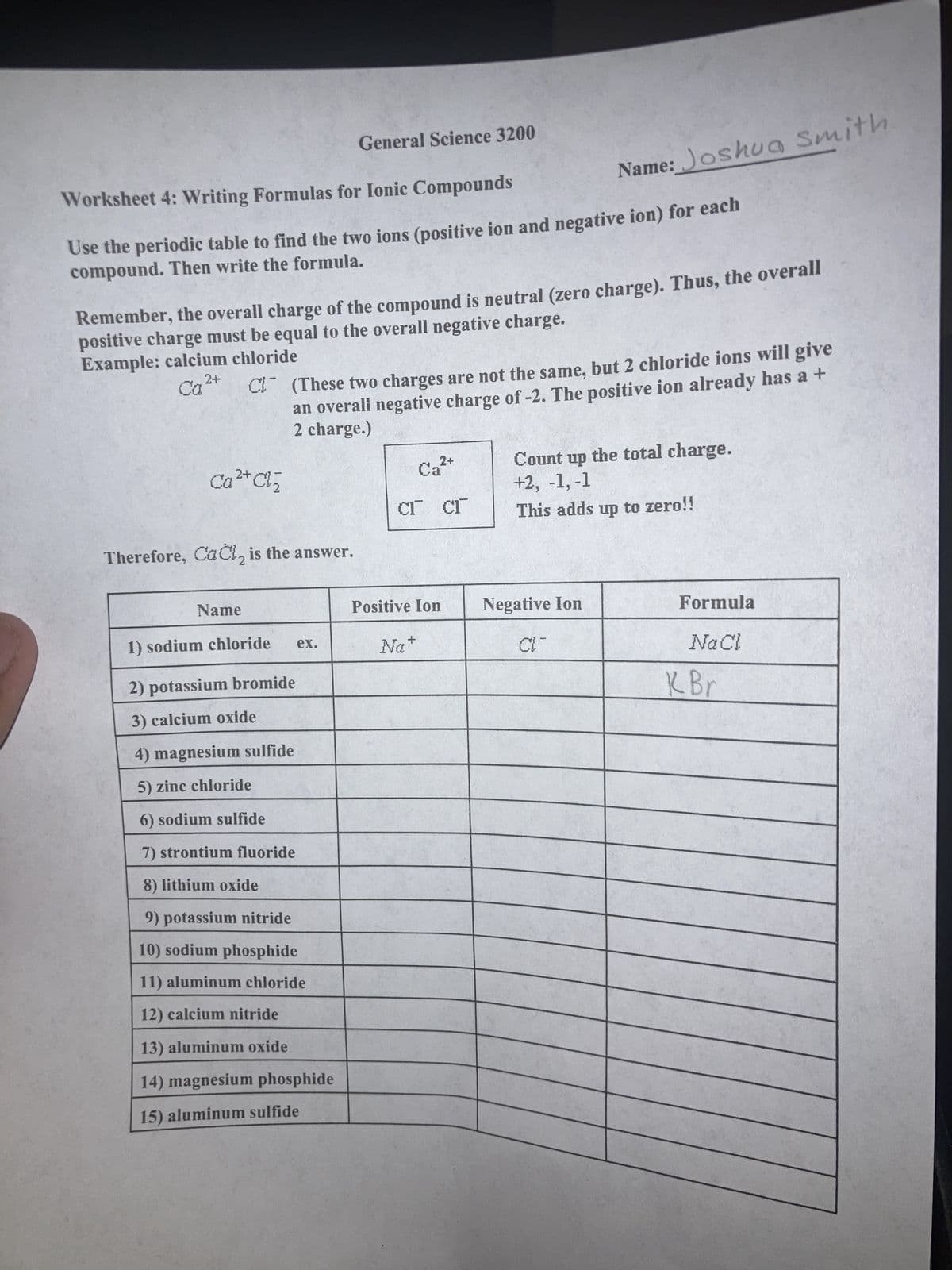
Transcribed Image Text:Worksheet 4: Writing Formulas for Ionic Compounds
Use the periodic table to find the two ions (positive ion and negative ion) for each
compound. Then write the formula.
Remember, the overall charge of the compound is neutral (zero charge). Thus, the overall
positive charge must be equal to the overall negative charge.
Example: calcium chloride
2+
Ca
2+
Ca²+ Cl₂
Therefore, CaCl₂ is the answer.
Name
General Science 3200
C (These two charges are not the same, but 2 chloride ions will give
an overali negative charge of -2. The positive ion already has a +
2 charge.)
1) sodium chloride
2) potassium bromide
3) calcium oxide
4) magnesium sulfide
5) zinc chloride
6) sodium sulfide
7) strontium fluoride
8) lithium oxide
9) potassium nitride
10) sodium phosphide
11) aluminum chloride
12) calcium nitride
13) aluminum oxide
14) magnesium phosphide
15) aluminum sulfide
ex.
Cr Cr
Ca²+
Positive Ion
Na
Name: Joshua Smith.
+
Count up the total charge.
+2, -1, -1
This adds up to zero!!
Negative Ion
CIT
Formula
Na Cl
K Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning