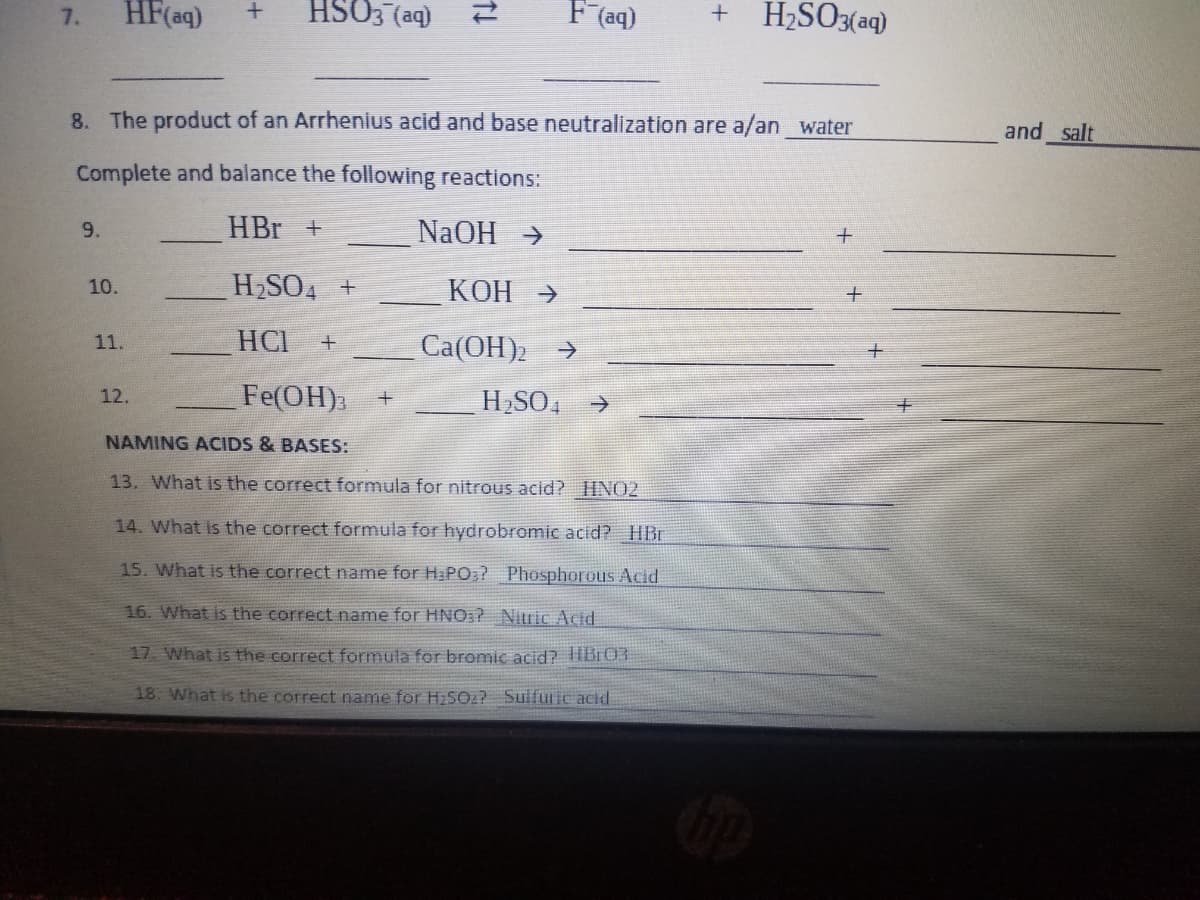
HF(aq) HSO3 (aq) F (aq) H2SO3(aq) 7. 8. The product of an Arrhenius acid and base neutralization are a/an water and salt Complete and balance the following reactions: HBr + NaOH > 9. H2SO4 + КОН > 10. 11. HCI Ca(OH)2 -> Fe(OH) 12. H2SO, > NAMING ACIDS & BASES: 13. What is the correct formula for nitrous acid? HNO2 14. What is the correct formula for hydrobromic acid? HBr 15. What is the correct name for Ha PO;? Phosphorous Acid 16. What is the correct name for HNO:? Nitric Acid 17. What is the correct formula for bromic acid? TIB:03 18 What is the correct name for H2SO:? Sulfuric acid
Analyzing Infrared Spectra
The electromagnetic radiation or frequency is classified into radio-waves, micro-waves, infrared, visible, ultraviolet, X-rays and gamma rays. The infrared spectra emission refers to the portion between the visible and the microwave areas of electromagnetic spectrum. This spectral area is usually divided into three parts, near infrared (14,290 – 4000 cm-1), mid infrared (4000 – 400 cm-1), and far infrared (700 – 200 cm-1), respectively. The number set is the number of the wave (cm-1).
IR Spectrum Of Cyclohexanone
It is the analysis of the structure of cyclohexaone using IR data interpretation.
IR Spectrum Of Anisole
Interpretation of anisole using IR spectrum obtained from IR analysis.
IR Spectroscopy
Infrared (IR) or vibrational spectroscopy is a method used for analyzing the particle's vibratory transformations. This is one of the very popular spectroscopic approaches employed by inorganic as well as organic laboratories because it is helpful in evaluating and distinguishing the frameworks of the molecules. The infra-red spectroscopy process or procedure is carried out using a tool called an infrared spectrometer to obtain an infrared spectral (or spectrophotometer).
According to Arrhenius theory,
acid is a substance that gives H+ ion on dissolving in the aqueous solution. It increases the concentration of H+ ions in the aqueous solution.
The base is a substance that ionizes OH– ion by dissolving in the aqueous solutions.
A neutralization reaction in which an acid and base quantitatively react together to form salt and water as products.
In a neutralization reaction, there is a combination of H+ ions and OH– ions which form water. A neutralisation reaction is generally an acid-base neutralization reaction.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images









